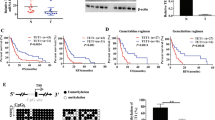
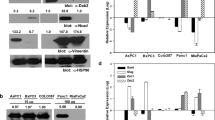
Abstract
The highly homeostasis-resistant nature of cancer cells leads to their escape from treatment and to liver metastasis, which in turn makes pancreatic ductal adenocarcinoma (PDAC) difficult to treat, especially the squamous/epithelial-to-mesenchymal transition (EMT)-like subtype. As the molecular mechanisms underlying tumour heterogeneity remain elusive, we investigated whether epigenetic regulation might explain inter-individual differences in the progression of specific subtypes. DNA methylation profiling performed on cancer tissues prior to chemo/radiotherapy identified one hypermethylated CpG site (CpG6882469) in the VAV1 gene body that was correlated with demethylation of two promoter CpGs (CpG6772370/CpG6772811) in both PDAC and peripheral blood. Transforming growth factor β treatment induced gene-body hypermethylation, dissociation of DNMT1 from the promoter, and VAV1 expression via SMAD4 and mutant KrasG12D. Pharmacological inhibition of TGFβ-VAV1 signalling decreased the squamous/EMT-like cancer cells, promoted nuclear VAV1 localization, and enhanced the efficacy of gemcitabine in prolonging the survival of KPfl/flC mice. Together, the three VAV1 CpGs serve as biomarkers for prognosis and early detection, and the TGFβ-VAV1 axis represents a therapeutic target.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Achyut BR, Yang L . Transforming growth factor-beta in the gastrointestinal and hepatic tumor microenvironment. Gastroenterology 2011; 141: 1167–1178.
Collazo J, Zhu B, Larkin S, Martin SK, Pu H, Horbinski C et al. Cofilin drives cell-invasive and metastatic responses to TGF-beta in prostate cancer. Cancer Res 2014; 74: 2362–2373.
Tufegdzic Vidakovic A, Rueda OM, Vervoort SJ, Sati Batra A, Goldgraben MA, Uribe-Lewis S et al. Context-specific effects of TGF-beta/SMAD3 in cancer are modulated by the epigenome. Cell Rep 2015; 13: 2480–2490.
Hezel AF, Deshpande V, Zimmerman SM, Contino G, Alagesan B, O'Dell MR et al. TGF-beta and alphavbeta6 integrin act in a common pathway to suppress pancreatic cancer progression. Cancer Res 2012; 72: 4840–4845.
Nakajima S, Doi R, Toyoda E, Tsuji S, Wada M, Koizumi M et al. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clin Cancer Res 2004; 10 (12 Pt 1): 4125–4133.
Ebert MP, Fei G, Schandl L, Mawrin C, Dietzmann K, Herrera P et al. Reduced PTEN expression in the pancreas overexpressing transforming growth factor-beta 1. Br J Cancer 2002; 86: 257–262.
Gore AJ, Deitz SL, Palam LR, Craven KE, Korc M . Pancreatic cancer-associated retinoblastoma 1 dysfunction enables TGF-beta to promote proliferation. J Clin Invest 2014; 124: 338–352.
Gaspar NJ, Li L, Kapoun AM, Medicherla S, Reddy M, Li G et al. Inhibition of transforming growth factor beta signaling reduces pancreatic adenocarcinoma growth and invasiveness. Mol Pharmacol 2007; 72: 152–161.
Ostapoff KT, Cenik BK, Wang M, Ye R, Xu X, Nugent D et al. Neutralizing murine TGFbetaR2 promotes a differentiated tumor cell phenotype and inhibits pancreatic cancer metastasis. Cancer Res 2014; 74: 4996–5007.
Katzav S . Vav1: A Dr. Jekyll and Mr. Hyde protein – good for the hematopoietic system, bad for cancer. Oncotarget 2015; 6: 28731–28742.
Katzav S, Martin-Zanca D, Barbacid M . vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. EMBO J 1989; 8: 2283–2290.
Qi Y, Kong FM, Deng Q, Li JY, Cui R, Pu YD et al. Clinical significance and prognostic value of Vav1 expression in Non-small cell lung cancer. Am J Cancer Res 2015; 5: 2491–2497.
Sebban S, Farago M, Rabinovich S, Lazer G, Idelchuck Y, Ilan L et al. Vav1 promotes lung cancer growth by instigating tumor-microenvironment cross-talk via growth factor secretion. Oncotarget 2014; 5: 9214–9226.
Grassilli S, Brugnoli F, Lattanzio R, Rossi C, Perracchio L, Mottolese M et al. High nuclear level of Vav1 is a positive prognostic factor in early invasive breast tumors: a role in modulating genes related to the efficiency of metastatic process. Oncotarget 2014; 5: 4320–4336.
Lindsey JC, Kawauchi D, Schwalbe EC, Solecki DJ, Selby MP, McKinnon PJ et al. Cross-species epigenetics identifies a critical role for VAV1 in SHH subgroup medulloblastoma maintenance. Oncogene 2015; 34: 4746–4757.
Garcia JL, Couceiro J, Gomez-Moreta JA, Gonzalez Valero JM, Briz AS, Sauzeau V et al. Expression of VAV1 in the tumour microenvironment of glioblastoma multiforme. J Neurooncol 2012; 110: 69–77.
Kataoka K, Nagata Y, Kitanaka A, Shiraishi Y, Shimamura T, Yasunaga J et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet 2015; 47: 1304–1315.
Bertagnolo V, Brugnoli F, Mischiati C, Sereni A, Bavelloni A, Carini C et al. Vav promotes differentiation of human tumoral myeloid precursors. Exp Cell Res 2005; 306: 56–63.
Brugnoli F, Lambertini E, Varin-Blank N, Piva R, Marchisio M, Grassilli S et al. Vav1 and PU.1 are recruited to the CD11b promoter in APL-derived promyelocytes: role of Vav1 in modulating PU.1-containing complexes during ATRA-induced differentiation. Exp Cell Res 2010; 316: 38–47.
Crespo P, Schuebel KE, Ostrom AA, Gutkind JS, Bustelo XR . Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature 1997; 385: 169–172.
Margolis B, Hu P, Katzav S, Li W, Oliver JM, Ullrich A et al. Tyrosine phosphorylation of vav proto-oncogene product containing SH2 domain and transcription factor motifs. Nature 1992; 356: 71–74.
Bustelo XR, Ledbetter JA, Barbacid M . Product of vav proto-oncogene defines a new class of tyrosine protein kinase substrates. Nature 1992; 356: 68–71.
Razanadrakoto L, Cormier F, Lauriente V, Dondi E, Gardano L, Katzav S et al. Mutation of Vav1 adaptor region reveals a new oncogenic activation. Oncotarget 2015; 6: 2524–2537.
Razidlo GL, Wang Y, Chen J, Krueger EW, Billadeau DD, McNiven MA . Dynamin 2 potentiates invasive migration of pancreatic tumor cells through stabilization of the Rac1 GEF Vav1. Dev Cell 2013; 24: 573–585.
Nolz JC, Gomez TS, Billadeau DD . The Ezh2 methyltransferase complex: actin up in the cytosol. Trends Cell Biol 2005; 15: 514–517.
Su IH, Dobenecker MW, Dickinson E, Oser M, Basavaraj A, Marqueron R et al. Polycomb group protein ezh2 controls actin polymerization and cell signaling. Cell 2005; 121: 425–436.
Gunawan M, Venkatesan N, Loh JT, Wong JF, Berger H, Neo WH et al. The methyltransferase Ezh2 controls cell adhesion and migration through direct methylation of the extranuclear regulatory protein talin. Nat Immunol 2015; 16: 505–516.
Fernandez-Zapico ME, Gonzalez-Paz NC, Weiss E, Savoy DN, Molina JR, Fonseca R et al. Ectopic expression of VAV1 reveals an unexpected role in pancreatic cancer tumorigenesis. Cancer Cell 2005; 7: 39–49.
Yang X, Han H, De Carvalho DD, Lay FD, Jones PA, Liang G . Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell 2014; 26: 577–590.
Jones PA . Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet 2012; 13: 484–492.
Grimmer MR, Farnham PJ . Can genome engineering be used to target cancer-associated enhancers? Epigenomics 2014; 6: 493–501.
Hou YC, Chao YJ, Tung HL, Wang HC, Shan YS . Coexpression of CD44-positive/CD133-positive cancer stem cells and CD204-positive tumor-associated macrophages is a predictor of survival in pancreatic ductal adenocarcinoma. Cancer 2014; 120: 2766–2777.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102: 15545–15550.
Bardeesy N, Aguirre AJ, Chu GC, Cheng KH, Lopez LV, Hezel AF et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci USA 2006; 103: 5947–5952.
Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016; 531: 47–52.
Thillainadesan G, Chitilian JM, Isovic M, Ablack JN, Mymryk JS, Tini M et al. TGF-beta-dependent active demethylation and expression of the p15ink4b tumor suppressor are impaired by the ZNF217/CoREST complex. Mol Cell 2012; 46: 636–649.
Ksionda O, Saveliev A, Kochl R, Rapley J, Faroudi M, Smith-Garvin JE et al. Mechanism and function of Vav1 localisation in TCR signalling. J Cell Sci 2012; 125 (Pt 22): 5302–5314.
Houlard M, Arudchandran R, Regnier-Ricard F, Germani A, Gisselbrecht S, Blank U et al. Vav1 is a component of transcriptionally active complexes. J Exp Med 2002; 195: 1115–1127.
Houlard M, Romero-Portillo F, Germani A, Depaux A, Regnier-Ricard F, Gisselbrecht S et al. Characterization of VIK-1: a new Vav-interacting Kruppel-like protein. Oncogene 2005; 24: 28–38.
Dong Z, Davidson D, Perez-Quintero LA, Kurosaki T, Swat W, Veillette A . The adaptor SAP controls NK cell activation by regulating the enzymes Vav-1 and SHIP-1 and by enhancing conjugates with target cells. Immunity 2012; 36: 974–985.
Reynolds LF, Smyth LA, Norton T, Freshney N, Downward J, Kioussis D et al. Vav1 transduces T cell receptor signals to the activation of phospholipase C-gamma1 via phosphoinositide 3-kinase-dependent and -independent pathways. J Exp Med 2002; 195: 1103–1114.
Miletic AV, Graham DB, Sakata-Sogawa K, Hiroshima M, Hamann MJ, Cemerski S et al. Vav links the T cell antigen receptor to the actin cytoskeleton and T cell activation independently of intrinsic guanine nucleotide exchange activity. PLoS One 2009; 4: e6599.
Saveliev A, Vanes L, Ksionda O, Rapley J, Smerdon SJ, Rittinger K et al. Function of the nucleotide exchange activity of vav1 in T cell development and activation. Sci Signal 2009; 2: ra83.
Razidlo GL, Magnine C, Sletten AC, Hurley RM, Almada LL, Fernandez-Zapico ME et al. Targeting pancreatic cancer metastasis by inhibition of Vav1, a driver of tumor cell invasion. Cancer Res 2015; 75: 2907–2915.
Li R, Wei F, Yu J, Li H, Ren X, Hao X . IDO inhibits T-cell function through suppressing Vav1 expression and activation. Cancer Bio Ther 2009; 8: 1402–1408.
Tsai JH, Yang J . Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev 2013; 27: 2192–2206.
Ehrich M, Nelson MR, Stanssens P, Zabeau M, Liloglou T, Xinarianos G et al. Quantitative high-throughput analysis of DNA methylation patterns by base-specific cleavage and mass spectrometry. Proc Natl Acad Sci USA 2005; 102: 15785–15790.
Krohn A, Diedler T, Burkhardt L, Mayer PS, De Silva C, Meyer-Kornblum M et al. Genomic deletion of PTEN is associated with tumor progression and early PSA recurrence in ERG fusion-positive and fusion-negative prostate cancer. Am J Pathol 2012; 181: 401–412.
Huang PH, Chen CH, Chou CC, Sargeant AM, Kulp SK, Teng CM et al. Histone deacetylase inhibitors stimulate histone H3 lysine 4 methylation in part via transcriptional repression of histone H3 lysine 4 demethylases. Mol Pharmacol 2011; 79: 197–206.
Huang PH, Chuang HC, Chou CC, Wang H, Lee SL, Yang HC et al. Vitamin E facilitates the inactivation of the kinase Akt by the phosphatase PHLPP1. Sci Signal 2013; 6: ra19.
Bai LY, Weng JR, Chiu CF, Wu CY, Yeh SP, Sargeant AM et al. OSU-A9, an indole-3-carbinol derivative, induces cytotoxicity in acute myeloid leukemia through reactive oxygen species-mediated apoptosis. Biochem Pharmacol 2013; 86: 1430–1440.
Acknowledgements
The authors acknowledge the assistance of Yi-Chen Chen, Pei-Rong Gu, and Hui-Ling Tung, as well as assistance from the following core facilities: the Clinical Medicine Research Center of National Cheng Kung University Hospital, the Animal Center of National Cheng Kung University, and the National Center for Genome Medicine (technical/bioinformatics). The authors would like to thank Drs Yi-Chuang Liao and Po-Min Chiang for pathological consultations and Dr Cindy Lee for manuscript editing. This work is supported by the Bi-institutional Collaborative Pancreatic Cancer Research grant from the College of Medicine of National Cheng Kung University to PJL and CSC; and grants MOST102-2320-B-006-050 and MOST104-2320-B-006-028- from the Ministry of Science and Technology, Taiwan, to PHH; MOHW104-TDU-B-211-124-003 from the Ministry of Health and Welfare, Taiwan, to YSS; and MOST 105-2321-B-001-064 from the Team of Excellent Research Program of the Ministry of Science and Technology, Taiwan, to CSC.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Huang, PH., Lu, PJ., Ding, LY. et al. TGFβ promotes mesenchymal phenotype of pancreatic cancer cells, in part, through epigenetic activation of VAV1. Oncogene 36, 2202–2214 (2017). https://doi.org/10.1038/onc.2016.378
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.378
This article is cited by
-
Epigenetic priming targets tumor heterogeneity to shift transcriptomic phenotype of pancreatic ductal adenocarcinoma towards a Vitamin D susceptible state
Cell Death & Disease (2024)
-
Identification of prognostic alternative splicing events in sarcoma
Scientific Reports (2021)
-
Vav1 Sustains the In Vitro Differentiation of Normal and Tumor Precursors to Insulin Producing Cells Induced by all-Trans Retinoic Acid (ATRA)
Stem Cell Reviews and Reports (2021)
-
Epigenetic silencing of AATK in acinar to ductal metaplasia in murine model of pancreatic cancer
Clinical Epigenetics (2020)
-
Vav1 mutations identified in human cancers give rise to different oncogenic phenotypes
Oncogenesis (2018)