Abstract
Both intracellular and extracellular heat shock protein-90 (Hsp90) family proteins (α and β) have been shown to support tumour progression. The tumour-supporting activity of the intracellular Hsp90 is attributed to their N-terminal ATPase-driven chaperone function. What molecular entity determines the extracellular function of secreted Hsp90 and the distinction between Hsp90α and Hsp90β was unclear. Here we demonstrate that CRISPR/Case9 knocking out Hsp90α nullifies tumour cells’ ability to migrate, invade and metastasize without affecting the cell survival and growth. Knocking out Hsp90β leads to tumour cell death. Extracellular supplementation with recombinant Hsp90α, but not Hsp90β, protein recovers tumourigenicity of the Hsp90α-knockout cells. Sequential mutagenesis identifies two evolutionarily conserved lysine residues, lys-270 and lys-277, in the Hsp90α subfamily that determine the extracellular Hsp90α function. Hsp90β subfamily lacks the dual lysine motif and the extracellular function. Substitutions of gly-262 and thr-269 in Hsp90β with lysines convert Hsp90β to a Hsp90α-like protein. Newly constructed monoclonal antibody, 1G6-D7, against the dual lysine region of secreted Hsp90α inhibits both de novo tumour formation and expansion of already formed tumours in mice. This study suggests an alternative therapeutic approach to target Hsp90 in cancer, that is, the tumour-secreted Hsp90α, instead of the intracellular Hsp90α and Hsp90β.
Similar content being viewed by others
Introduction
The heat shock protein-90 (Hsp90) family proteins (Hsp90α and Hsp90β) are among the most abundantly expressed proteins in almost all nucleated cells and are historically known as ATPase-driven molecular chaperones.1 Hsp90α and Hsp90β together make up 2–3% of the total proteins in normal cells and up to 7% in certain tumour cells,2 with a 2:1 ratio of Hsp90α to Hsp90β.3 Inside the cells, Hsp90 acts to maintain the stability and functionality of numerous so-called ‘client proteins’ in an ATPase-dependent manner.4 Many of the client proteins are critical components of the cellular signalling pathways that regulate cell survival, metabolism and growth.5, 6, 7, 8
Studies of the past few years, in particular, have demonstrated importance of the cell surface-bound or -secreted Hsp90α protein as a novel pro-motility factor in normal cells during tissue repair9 and a pro-invasion factor during tumour cell invasion.10 It is clear now that normal cells secrete Hsp90, especially Hsp90α, under stress such as hypoxia, ultraviolet light, ionizing radiation, free radicals and tissue injury. Tumour cells, including so far breast, colon, bladder, prostate, skin, liver and bone, constitutively secrete Hsp90α and Hsp90β.11 A well-characterized upstream regulator of Hsp90 secretion in both normal and tumour cells is the hypoxia-inducible factor-1 alpha (HIF-1α), which is undetectable in normal cells under normoxia (physiological) conditions and constitutively (even under normoxia) overexpressed in >50% of all invasive tumours in humans.12 HIF-1α mediates hypoxia-triggered Hsp90 secretion via the unconventional exosome secretion pathway.13, 14, 15 The reported mechanisms of action by extracellular Hsp90α include binding and activating secreted MMP2,9, 16 interacting with the HER-2 tyrosine kinase receptor and Cdc37,17, 18 associating with lysyl oxidase 2-like protein (LOXL2);19 regulating the function of the methyltransferase of the polycomb repressor complex, EZH2,19 and via the ‘HIF-1α > Hsp90α secretion > LRP-1 receptor’ pathway.10, 20 Moreover, recent studies showed that the plasma level of Hsp90α correlates with the pathologic stage of cancer in patients.21, 22
In the study herein, we investigated whether secreted Hsp90α is essential or complementary during tumour progression, how secreted Hsp90α differs from its intracellular counterpart and what molecular entity grants Hsp90α, but not Hsp90β, its extracellular function. Our results show that the secreted form of Hsp90α is essential for promoting tumour invasion in vitro and tumour formation and metastasis in vivo. Two evolutionarily conserved lysine residues, lys-270 and lys-277, which are only present in the Hsp90α subfamily members, are crucial for the tumourigenic activity of secreted Hsp90α. A newly constructed monoclonal antibody, 1G6-D7, targeting the dual lysine region in Hsp90α, effectively inhibits both de novo tumour formation and continued growth of already formed tumours in mice.
Results
Distinct roles for Hsp90α and Hsp90β in the tumour cell survival
We focused on the highly invasive and metastatic breast cancer cell line, MDA-MB-231.23 These cells show a deregulated expression of HIF-1α, a ~3.5% steady-state Hsp90 protein within the cells and a constitutive HIF-1α-driven secretion of Hsp90α and Hsp90β.2 This cell model has widely been utilized in studies of secreted Hsp90.2, 16, 23, 24, 25, 26
The CRISPR/Cas9 technology27 was utilized to knockout Hsp90α and Hsp90β genes in MDA-MB-231 cells. As shown in Figure 1A, a small fraction of the cells survived the two rounds of drug selection for Hsp90α gene knockout (panel c vs panels a and b). In contrast, the cells that were subjected to similar selection procedures for Hsp90β gene knockout stopped proliferating, detached and ultimately died (panel f vs panels d and e). However, among the 47 individual clones isolated and screened for evidence of Hsp90α knockout, we found only two clones that showed complete absence of Hsp90α protein. To investigate the possible reason for such an unexpected lower number, we found that there are up to 20 Hsp90α pseudogenes with 65–100% identity to the wild-type Hsp90α gene in the human genome (UCSC Genome Bioinformatics, see Supplementary Figure S1A). Based on this information, the chance for the CRISPR/Cas9 design of ours to hit both alleles of the authentic Hsp90α gene in the same cell was several magnitudes lower than expected. Nonetheless, as shown in Figure 1B, the two independently derived Hsp90α-knockout cell clones, KO-α-#1 and KO-α-#2, showed complete absence of Hsp90α protein (panel g, lanes 3 and 4), in comparison with the control cells (lane 1) and the cells with Hsp90α downregulation by a lentivirus-delivered short hairpin RNA against Hsp90α (lane 2). In the Hsp90α- knockout and downregulated cells, the cellular Hsp90β level was significantly elevated (panel h, lanes 2, 3 and 4, vs lanes 1 and 2), which explains the less changes in the total Hsp90 proteins following downregulation of Hsp90α (panel i). The specificity of the anti-Hsp90α and anti-Hsp90β antibodies was verified by purified recombinant Hsp90α and Hsp90β proteins in western blot (Figure 1D), in which the antibodies showed little cross reactions. As expected, Hsp90α secretion was no longer detectable from the Hsp90α-knockout cells, in comparison with the control cells (Figure 1D, panel l, lane 5 vs lane 4). The amount of secreted Hsp90β remained unchanged (panel m, lane 5 vs lane 4). Human recombinant Hsp90 (α and β) proteins were included as controls (lanes 1–3). The specificity of Hsp90α knockout, instead of off target effects, was further confirmed by sequencing the Hsp90α gene and gene rescue experiments in the Hsp90α-knockout cells. We found that the CRISPR/Cas9 created a break in Hsp90α gene that deleted amino acids 132–138, followed by a premature stop codon (see Supplementary Figure S1C vs Figure 1B, as indicated by arrows). More convincingly, ectopically expressed Hsp90α-wt or ATPase-defective Hsp90α (Hsp90α-D93N), but not Hsp90β, gene rescued the motility and invasion defects of the Hsp90α-knockout cells (see Supplementary Figure S2).
Distinct roles for Hsp90α and Hsp90β in cancer cell survival and tumourigenicity. (A) Survival of MDA-MB-231 cells under drug selection following CRISPR-Cas9 Hsp90α gene (panels a, b and c) or Hsp90β gene (panels d, e and f) knockout was measured by counting the live cells over time (n=4). (B) Two Hsp90α-knockout clones (KO-α#1 and KO-α#2) showed complete absence of Hsp90α protein (panel g, lanes 3 and 4), in comparison with either short hairpin RNA (shRNA)-Hsp90α knockdown (lane 2) or the native MDA-MB-231 (lane 1) cells. (C) Specificity of the anti-Hsp90α and Hsp90β antibodies was confirmed by using the recombinant proteins in the Western. (D) Hsp90α gene knockout blocks Hsp90α (panel l), but not Hsp90β (panel m), secretion. (E) Growth curves of the native, shRNA-Hsp90α-knockdown (shRNA-α) and Hsp90α-knockout (KO-α-#1) MDA-MB-231 cells in the absence or presence of 10% foetal bovine serum (FBS). (F) Effect of Hsp90α gene on signalling in response to epidermal growth factor or TGFα.
In contrast to an essential role for Hsp90β in the tumour cell survival, we found that the proliferation profiles of either Hsp90α-knockdown or knockout MDA-MB-231 cells were indistinguishable from their unperturbed native counterpart (Figure 1E). Furthermore, Hsp90α knockout did not cause significant alterations in signal transduction. As shown in Figure 1F, constitutive ERK1/2 phosphorylation (panel n), transforming growth factor alpha (TGFα)- and epidermal growth factor-stimulated p38 phosphorylation (panel o) and constitutive PRAS40 phosphorylation (at threonine-246) (panel q) remained unchanged in the Hsp90α-knockout cells, in comparison with the native MDA-MB-231 cells (lanes 4–6 vs lanes 1–3). TGFα-, but not epidermal growth factor-, stimulated Akt phosphorylation (S-473) was reduced in Hsp90α-knockout cells (panel p, lane 5 vs lane 2). Although the reason remains to be investigated, it may be caused by the previously reported difference between TGFα and epidermal growth factor signalling in stimulating Hsp90α secretion.10 Nonetheless, these findings are consistent with the outcomes of genetic studies that Hsp90α-knockout mice were normal and Hsp90β knockout was lethal.28, 29, 30
Hsp90α knockout selectively eliminates tumour cell motility and invasiveness
The Hsp90α knockout, however, eliminated the tumour cell’s ability for motility and invasion. We used the colloidal gold migration assay initially established by Albrecht-Buehler31 and modified by us.32 To quantitate the data, 15 randomly selected cell migration fields in each well are analysed by the computer using NIH Image 1.6 program (National Institute of Health, Bethesda, MD, USA), which calculates the percentage of the field area consumed by cell migration tracks over the total field area viewed under the microscope in triplicate wells and gives rise to the so-called migration index (MI).33 Both photographs of the migration tracks and the MIs are used as evidence of cell motility. As shown in Figure 2A, the native MDA-MB-231 cells exhibited a constitutively high motility even under serum-free conditions (panel a vs panel b) (dotted circles indicated the average size of the migration tracks under those conditions). In comparison, the non-transformed breast epithelial cell line, HBL-100, and primary human keratinocytes showed a basal level of cell motility in the absence of serum (panels c and e) and a markedly increased motility in the presence of serum (panels d and f). Interestingly, as shown in Figure 2B, the Hsp90α-knockout cells, KO-α-#1 and KO-α-#2, completely lost their intrinsic motility (panels h and k vs panel g). This loss of motility was specifically due to the absence of secreted Hsp90α, as supplementation of the cells with Hsp90α protein fully rescued their motility (panels i and l vs panels h and k). In contrast, Hsp90β was unable to rescue the defect (panels j and m).
Hsp90α knockout eliminates motility and invasiveness of the tumour cells. Serum-starved cells were subjected to colloidal gold motility (5000 cells per well) and the Matrigel invasion (20 000 cells per well) assays. (A) The migration tracks of MDA-MB-231, HBL-100 and human keratinocytes without (−) or with (+) 10% foetal bovine serum were quantitated by a computer-assisted analysis as MI (Materials and methods), as shown. (B) The motility measurements of the native MDA-MB-231, KO-α-#1 and KO-α-#2 cells in the absence (−) or presence (+) of human recombinant Hsp90α or human recombinant Hsp90β protein stimulation. (C) Matrigel invasion assay of the indicated cell lines with or without added human recombinant Hsp90α or human recombinant Hsp90β protein and the results quantitated as percentage of the invaded cells over the total number of seeded cells (Inv. %). (D) Evidence for FPLC-purified human recombinant Hsp90α and human recombinant Hsp90β proteins used in the above rescue experiments. (E) Evidence for downregulation of LRP-1 receptor in MDA-MB-231 cells by a lentivirus-delivered short hairpin RNA. (F) Matrigel invasion assay of LRP-1-downregulated cells in the absence or presence of human recombinant Hsp90α protein. Data were representative of multiple (n⩾ 4) independent experiments and represented as mean±s.e.m. P<0.05.
Similar results were obtained for the loss of the cells’ ability to invade a Matrigel membrane. As shown in Figure 2C, the control cells line, HBL-100, was non-invasive (panel n). In contrast, MDA-MB-231 cells showed a strong invasiveness (panel o). Both KO-α-#1 and KO-α-#2 cells, however, lost their invasiveness (panels p and s). Supplementation of the cells with human recombinant Hsp90α (panels q and t), but not human recombinant Hsp90β, (panels r and u) protein fully recovered the invasiveness of the cells. The purified human recombinant Hsp90α and human recombinant Hsp90β proteins are shown in Figure 2D. To ascertain that the rescue by human recombinant Hsp90α is through the extracellular signalling via the LRP-1 pathway as we have previously shown,2, 3, 10, 20 we downregulated the cell surface LRP-1 in MDA-MB-231 cells, as shown in Figure 2E (lane 2 vs lane 1) and found that downregulation of LRP-1 abolished the invasiveness of the cells (panel w vs panel v), just like KO-α-#1 and KO-α-#2 cells. Moreover, unlike the Hsp90α-knockout cells, supplementation of the LRP-1-downregulated cells with human recombinant Hsp90α protein was unable to rescue the invasion defect (panel x), because the LRP-1 receptor acts downstream of secreted Hsp90α. Finally, the rescue was extracellular, as we were unable to detect any His-tagged Hsp90α or Hsp90β protein from the cytosol fraction of the cells by anti-His tag antibodies.
Evolutionarily conserved dual lysines motif determines the extracellular function of Hsp90α
Although the N-terminal ATPase determines the chaperone function of intracellular Hsp90 proteins, we wanted to identify the molecular entity that determines the extracellular function of secreted Hsp90α. Based on the fact that extracellular Hsp90β does not share the same function of extracellular Hsp90α, we hypothesized that this molecular entity should only be present in Hsp90α, but not Hsp90β. We undertook two steps to complete the study, sequential deletion and site-directed mutagenesis, and used the rescue of the motility of Hsp90α-knockout MDA-MB-231 cells. As shown in Figure 3a, the starting fragment was the 115-amino-acid peptide, called F-5, which retains the full extracellular pro-motility activity of the 732-amino-acid full-length Hsp90α.34 The deletion mutagenesis approach allowed us to narrow the pro-motility activity-containing peptide from F-5 down first to a 54-amino-acid fragment, F-6, and to a final 27-amino-acid fragment, called F-8, the smallest peptide that was able to rescue the motility of the Hsp90α-knockout MDA-MB-231 cells (see the MI). The F-8 was also small enough for substituting the individual amino acids by site-directed mutagenesis. When we lined the 27-amino-acid sequence of F-8 (F-8α) of Hsp90α with the corresponding 27-amino-acid sequence from Hsp90β, F-8β, as shown in Figure 3b, we found eight amino acids within F-8α (green) that are substituted by eight different amino-acid residues in F-8β (red). This finding suggested that the molecular entity of Hsp90α must locate within the eight variant amino-acid residues that distinguish Hsp90α from Hsp90β for having the extracellular function.
Lysine-270 and lysine-277 determine extracellular function of Hsp90α mutagenesis to identify the essential amino-acid residues for extracellular pro-motility activity of human Hsp90α. (a) Deletion mutagenesis narrowed the pro-motility activity of the 115-amino-acid F-5 fragment down to a 27-amino-acid peptide, F-8 fragment. (b) Comparison of F-8 from Hsp90α (F-8α) with the corresponding sequence of Hsp90β, F-8β, shows eight amino acids in F-8α (green) substituted with variant amino acids in F-8β (red). (c) Eight synthetic peptides with each of the eight amino acids in F-8α replaced with each of the corresponding amino-acid residues from F-8β (in red) were screened for their ability to rescue the motility defect of Hsp90α-knockout MDA-MB-231 cells, with F-8α and F-8β as positive and negative controls respectively. Quantitation of the cell motility is shown as MI (%). (d) K-270 and K-277 and their corresponding amino acids, G-262 and T-269, in Hsp90β were emphasized as focus of further studies.
To test this hypothesis, as shown in Figure 3c, eight synthetic peptides were produced in which each of the eight variant amino acids in F-8α was replaced with the corresponding amino-acid residue from F-8β (in red). These peptides with a single-amino-acid substitution were screened for their ability to rescue the motility defect of the Hsp90α-knockout MDA-MB-231 cells. Substitution of the lysine-270 or the lysine-277 markedly reduced the ability of the two peptides to rescue the cell motility (MIs in red). The remaining six peptides with other amino-acid substitutions were all able to rescue the migration (MIs in green). Therefore, as shown in Figure 3d, lys-270 and lys-277 in Hsp90α are the potential molecular entity that determines its extracellular function. More intriguingly, these two lysine residues were found to be evolutionarily conserved in the Hsp90α subfamily genes from zebrafish to humans and less conserved in the single Hsp90 gene species, including fly, worm and Yeast (see Supplementary Figure S3A). Similarly, the corresponding gly-262 and thr-269 substitutions are highly conserved in the Hsp90β subfamily genes throughout the evolution (see Supplementary Figure S3B).
To ultimately confirm the importance of lysine-270 and lysine-277, the mutations were introduced into the full-length Hsp90α and Hsp90β genes and their effects tested. We reasoned that mutations at lysine-270 and lysine-277 in Hsp90α would nullify its extracellular function. In reverse, we expected that substitutions of glycine-262 and threonine-269 in Hsp90β with two lysine residues would grant the Hsp90β the ability to promote invasion and tumour formation similar to Hsp90α. As schematically shown in Figure 4A, we substituted lysine-270 and lysine-277 in full-length Hsp90α with the two corresponding residues, glycine-262 and threonine-269, from Hsp90β, to create the Hsp90α-K270G/K277T mutant. We also generated a random and nonspecific mutant, Hsp90α-D271K, as a control for specificity. In reverse, as shown in Figure 4B, we replaced glycine-262 and threonine-269 in Hsp90β with two lysine residues to create the Hsp90β-G262K/T269K mutant. A random mutant of Hsp90β-K263D was created as a control. The six purified wild-type and mutant proteins of full-length Hsp90α and Hsp90β proteins are shown in Figure 4C (lanes 4–9), with known amounts of pure bovine serum albumin as quantitative comparisons (lanes 1–3).
Lysine-270 and lysine-277 substitutions convert Hsp90β to Hsp90α-like protein to promote cancer cell motility and invasion. (A) A schematic representation of lysine-270 and lysine-277 in full-length Hsp90α and creations of the Hsp90α-K270G/K277T and Hsp90α -D271K mutants. (B) A schematic representation of glycine-262 and threonine-269 in full-length Hsp90β and creations of the Hsp90β-G262K/T269K and Hsp90β-K263D mutants. (C) Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) with Coomassie Brilliant Blue staining of FPLC-purified recombinant Hsp90α and Hsp90β proteins. Commercial bovine serum albumin (BSA) is included as a comparison for relative quantities. (D) Circular dichroism (CD) revealed the secondary structural profiles of Hsp90α, Hsp90β, and their mutant proteins. (E) Rescue of the invasion defect of the KO-α-#1 cells (panel b vs panel a) by supplementation with wild-type Hsp90α (panel c), Hsp90α-G/T mutant (panel d), Hsp90α-D271K mutant (panel e), wild-type Hsp90β (panel f), Hsp90βK/K mutant (panel g) and Hsp90β-K263D mutant (panel h) (30 μg/ml). Quantitation of invasion (Inv. %) is included.
Before functional studies, we examined whether the point mutations affect the global structure of the Hsp90 proteins by circular dichroism, which provides the secondary structural profiles of these proteins. As shown in Figure 4D, the circular dichroism spectra of Hsp90α and Hsp90β were characteristic of folded proteins containing a mix of secondary structure elements. Hsp90β exhibited a slightly lower ellipticity at 222 nm than Hsp90α, revealing a higher helical content. Most importantly, the mutant variants of Hsp90α and Hsp90β were structurally indistinguishable from their wild-type counterparts. In addition, these mutations did not affect the ATPase activity of the proteins (see Supplementary Figure S5A). To test the ability of these proteins to rescue the invasiveness of the Hsp90α-knockout cells, as shown in Figure 4E, we found that the wild-type Hsp90α and the nonspecific Hsp90α-D271K mutant proteins remained capable of rescuing the invasion defect of the cells (panels c and e vs panel b). However, the Hsp90α-K270G/K277T mutant lost the rescue ability (panel d). As expected, the wild-type Hsp90β protein showed little rescue effect (panel f). Intriguingly, the Hsp90β-G263K/T269K (panel g), but not the nonspecific Hsp90β-K263D (panel h), mutant acted like the wild-type Hsp90α protein and became able to rescue the invasion defect of the Hsp90α-knockout cells. These findings established a crucial role for the evolutionarily conserved dual lysine residues in the extracellular function of Hsp90α in vitro. These dual lysine residues selectively control the pro-motility activity of Hsp90α, but not its binding to the LRP-1 receptor, as an increasing amount of the Hsp90α-K270G/K277T mutant acted as dominant-negative molecule to compete off the function of the tumour cell-secreted Hsp90α (see Supplementary Figure S4).
Requirement of lys-270 and lys-277 for secreted Hsp90α’s tumourigenicity in vivo
We tested the ability of recombinant Hsp90α-wt, Hsp90αK270G/K277T, Hsp90β-wt and Hsp90β-G262K/T269K proteins to rescue the lost tumourigenicity of the Hsp90α-knockout MDA-MB-231 cells in mice. As shown in Figure 5A, the parental MDA-MB-231 cells formed tumours in four of the five mice (panel a) at the mammary fat pad (the recognized orthotopic site for breast cancer studies) on day 28 when the entire experiment ended (when a tumour reaches the IACUC limitation of 1.5 cm in size). The tumours were excised and measured as tumour volume (TV, mm3) (panel b) (see Materials and methods section). Interestingly, we found no tumour formation in five of the five mice in the group injected with the KO-α-#1 cells (panels c). Histological analysis of the excised tumours or the corresponding tissue from the injection site confirmed the results (Figure 5B, panel c’ vs panel a’). All the tumours in various sizes from the four parental MDA-MB-231 cell-injected mice metastasized to the lung (panel b’), one of the two major sites for metastasis by MDA-MB-231 cells, as previously reported.35 In contrast, we did not detect cancer metastasis to the lung in any of the KO-α cell-injected mice (panel d’).
Tumour formation and metastasis in mice requires Lysine-270 and lysine-277 of Hsp90α. (A) Results of a representative experiment showing tumour formation of injected (5 × 106) native MDA-MB-231 (panels a and b), KO-α-#1 cells alone (panel c) or KO-α-#1 cells supplemented with wild-type human recombinant Hsp90α (panels d and e), or with human recombinant Hsp90α-G/T mutant (panel f) or wild-type human recombinant Hsp90β (panel g) or with human recombinant Hsp90β-K/K mutant (panel h) protein to the mammary fat pad of nude mice (n=5 per group). The detected tumour formation was indicated by dotted circles and excised tumours measured for their Tumour Volume (TV, mm3). (B) Histological (H&E) analyses of the tumour, mammary fat pad tissue and the lung cryosections from non-tumour and tumour-containing mice as indicated. This experiment was repeated three times (n=4 or 5 per group). hr, human recombinant; T (red), tumour; N (blue), normal tissue; 5/5, five out of the five mice; Data are represented as mean±s.e.m. P<0.05.
However, co-injection of the KO-α cells with the Hsp90α-wt protein fully rescued the cells’ ability to form tumours in mice (panels d and e). In addition, under these conditions, the averaged TV of the tumours was even larger than those by the parental MDA-MB-231 cells (panel e vs panel b). As expected, the tumourigenic activity of Hsp90α-wt protein does not require the intrinsic ATPase, as the ATPase-defective mutant, Hsp90α-D93N, was equally capable of promoting tumour formation and metastasis (see Supplementary Figure S5B). In contrast, the Hsp90αK270G/K277T mutant protein showed undetectable rescue effect (panel f), providing evidence that lysine-270 and lysine-277 are crucial for extracellular Hsp90α’s tumourigenic activity in vivo. As expected, co-injection of the KO-α cells with Hsp90β-wt protein did not promote tumour formation (panel g). Interestingly, although the Hsp90β-G262K/T269K mutant protein acted as an Hsp90α-like molecule in the in vitro motility and invasion assays (Figures 3 and 5), it was unable by itself to recapitulate the full tumourigenic activity of Hsp90α-wt protein in vivo (panel h). Histological analysis confirmed that addition of Hsp90α-wt protein promoted the KO-α cells to form tumours at the mammary fat pad (panel e’) and to metastasize to the lung (panel f’). In contrast, we were unable to detect tumour formation and metastasis by the KO-α cells supplemented with the Hsp90αK270G/K277T mutant protein (panels g’ and h’) or Hsp90β-wt protein (panels i’ and j’). The tumourigenic effect of Hsp90β-G262K/T269K mutant protein (panels k’ and l’) was weak or undetectable, in comparison with the Hsp90α-wt protein (panels e’ and f’). This finding indicates the complexity of the tumour environment and tumour progression in vivo. We speculate that the lysine-270 and lysine-277 residues work with an additional molecular element(s) in Hsp90α to accomplish its tumourigenic function in the tumour environment in mice.
Endogenously secreted Hsp90α is required for tumour formation and metastasis
To directly prove that tumour-secreted Hsp90α has an essential role in promoting tumour formation and metastasis in mice, we developed lines of monoclonal antibody targeting the region that includes the two lysine residues in Hsp90α, as schematically shown in Figure 6a. Two hybridoma lines, 1G6-D7 and 5C4-D4, were isolated from screening >800 cell clones (see Supplementary Methods). Purified IgG from conditioned media of the cells are shown in Figure 6b (lanes 2 and 3), with commercial mouse IgG as control (lane 1). Using 35 synthetic (15-amino-acid) peptides in competitive binding assays (ELISA), the epitopes of 1G6-D7 and 5C4-D4 antibodies in F-5 were mapped to TKPIWTRNP for 1G6-D7 and to VKHFSVEGQ for 5C4-D4 (Figure 6c). Under the similar conditions, 1G6-D7, whose epitope is closer to the two lysine residues exhibited significantly stronger binding and neutralizing activity to native secreted Hsp90α from MDA-MB-231 cells than 5C4-D4 (see Supplementary Figure S6A). We therefore focused on 1G6-D7. As expected, 1G6-D7 blocked parental MDA-MB-231 cell invasion in a dose-dependent manner (see Supplementary Figure S6B, panels b and c vs panel a), whereas IgG control did not show any inhibition (panel d). To confirm the binding specificity of 1G6-D7, the addition of F-5 peptide reversed the inhibition (see Supplementary Figure S6B, panels f and g vs panel e), whereas the addition of F-5 alone slightly enhanced the cell invasion (panel h vs panel a), as expected.
Development of secreted Hsp90α-neutralizing monoclonal antibodies against the dual lysine region of human Hsp90α. (a) A schematic illustration of the antigen (F-5) location in reference to the dual lysine residues in full-length Hsp90α. (b) IgG was purified by protein-G Sepharose affinity chromatography from two hybridoma clones, 1G6-D7 and 5C4-D4, and analysed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) with coomassie brilliant blue staining. Regular mouse IgG was used as a control. (c) 1G6-D7 (IgG1κ) and 5C4-D4 (IgG2aκ) are mapped to bind to TKPIWTRNP and VKHFSVEGQ, respectively, within the antigen.
More encouragingly, co-injection of 1G6-D7 with the tumour cells into nude mice prevented tumour formation and tumour metastasis to lung. As shown in Figure 7A, the parental MDA-MB-231 cells formed large tumours in five of the five mice that were co-injected with a control mouse IgG (panels a and b). Co-injection with 1G6-D7 almost completely prevented the tumour formation (panels c and d). When TV values (n=5, per experiment) were measured on live mice over the period of 4 weeks, as shown in Figure 7B, we observed that the tumours in 1G6-D7-injected mice initially grew for the first 2 weeks (green arrow), but ultimately failed to catch up the same pace as their parental counterparts in mice co-injected with a control IgG. Histological analysis confirmed the lack of tumours in 1G6-D7-treated mice (Figure 7C, panel c vs panel a). The lung specimens excised from mice co-injected with control mouse IgG showed metastasized tumour cells (panel b). In contrast, no detectable tumour metastasis to lung was found in mice co-injected with 1G6-D7 (panel d).
1G6-D7 blocks tumour formation and metastasis in vivo. Although 1G6-D7 inhibits tumour cell invasion in vitro (see Supplementary Figure S6), it also works in vivo. (A) A representative experiment showing tumour formation of injected native MDA-MB-231 cells (5 × 106) in mice (n=3 or 5 per group) that were intravenously administered with either control mouse IgG or 1G6-D7 (5 mg/kg/injection) (Methods). (B) Measurement of tumour volumes on live mice over 4 weeks. (C) H&E staining of the primary tumour/tissue (panels a and c) or the lungs (panels b and d) sections from IgG control- or 1G6-D7-treated mice as indicated. T, tumour; N, normal tissue. 5/5, five out of five mice per group. This experiment was repeated three times (n=4 or 5 mice per group). Data are represented as mean±s.e.m. P<0.05.
1G6-D7 blocks continued growth of already formed tumours in mice
Clinically, a patient is treated with antitumour therapeutics, because he/she has been diagnosed with an already formed tumour. A clinically relevant question was whether 1G6-D7 is able to inhibit the growth of an already formed tumour under the same conditions as described previously. To study this question, mice were injected with the parental MDA-MB-231 cells and tumours were allowed to grow to an average size of 60 mm3, as standardized previously by others,36, 37, 38 before treatment with 1G6-D7 every 48 h. The TV values (mm3) were measured every 5 days on the live animals during the experimental period of 35 days. The final TV measurement of excised tumours was carried out on day 36, when all animals were killed. As shown in Figure 8A, all tumours grew equally up to day 5 before treatments. Interestingly, the tumours treated with 1G6-D7 from the day 5 continued to grow, but at a significantly slower rate over the experimental period of time (red line) than the tumours treated with either vehicle alone (orange line) or with vehicle plus a control mouse IgG (green line) during the same period of time. When the experiments ended on day 35 (because of IACUC regulation), tumours excised from the site of mammary fat pad on day 36 and the TV values measured. As shown in Figure 8B, under the (may not be perfect) treatment conditions, 1G6-D7 reduced the tumour size for an average 35%. Taken together, the inhibitory effect of 1G6-D7 on both tumour formation and (already formed) tumour growth provides evidence that secreted Hsp90α is potentially a promising therapeutic target in certain tumours that depend upon secreted Hsp90α for progression.
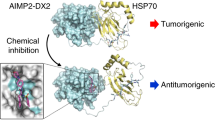
1G6-D7 inhibits expansion of pre-formed tumours in vivo. (A) A representative experiment showing tumour formation by injected parental MDA-MB-231 cells (5 × 106) over 35 days. On day 5 with an average tumour size of 60 mm3, either vehicle, control mouse IgG or 1G6-D7 was injected via IV (5 mg/kg) and around the tumour site (125 μg per injection). Measurement of the tumour volumes on live mice (n=4 or 5) was carried out every 5 days. This experiment was repeated twice. Data are represented as mean±s.e.m. P<0.05. (B) The images of the mice and the TV measurement of the excised tumours from the mice on day 36 (the s.d. represents three independent measurements with different angles of a tumour). (C) Based on the previously evaluated crystal structures of Hsp90α’s NTD (green) and MD (blue) plus CTD (red) domains and their fit into a cryoEM map of full-length Hsp90α bound to Hop (MODELLERv9.14). Lysine-270 and lysine-277 are located in the unstructured linker region (LR) between the NTD and MD domains, called F-5. Inhibitors, such as 1G6-D7, targeting the dual lysine residues (larger box) could block secreted Hsp90α-triggered tumourigenesis.
Discussion
In this study, we found that Hsp90α and Hsp90β do not functionally compensate for each other. Hsp90β, but not Hsp90α, is critical for the cell viability. The secreted form of Hsp90α, but not Hsp90β, is responsible the tumour cells’ abilities to migrate, invade and form tumours in mice. The extracellular function of Hsp90α is regulated via N-terminal ATPase-independent mechanisms that involve two critical lysine residues in the linker region in Hsp90α but not in Hsp90β. Monoclonal antibody, 1G6-D7, showed a complete inhibition of tumour formation, when it was co-injected with the tumour cells and a partial inhibition of the continued growth of already formed tumours. Although it is possible that the dosage and treatment frequency by 1G6-D7 may not be optimal, results of the proof of principle studies are consistent with the most clinical data of cancer treatments, the earlier the treatment, the better efficacy for an antitumour therapy. Thus, tumour-secreted Hsp90α could be a safe and effective target for antitumour therapeutics and the dual lysine motif is the epitope for developing new therapeutics.
The dual lysine motif, lysine-270 and lysine-277, is evolutionarily conserved from yeast to humans in the Hsp90α subfamily. In the Hsp90β subfamily, the two lysines are substituted with other amino acids, which correlate with the lack of such extracellular activities as Hsp90α has. Substitutions with lysines at the two corresponding amino-acid positions in Hsp90β are sufficient to grant Hsp90β with the similar pro-motility and pro-invasion activities of the Hsp90α in vitro. Interestingly, the lysine substitutions alone are not sufficient to convert Hsp90β into an Hsp90α-like protein that promotes tumour formation and metastasis in mice. The latter data suggest that an additional molecular element(s) in Hsp90α is required to work together with the dual lysine residues in the tumour microenvironment to promote tumourigenesis. This current finding is as schematically depicted in Figure 8C, in which inhibitors that target the dual lysine residues in secreted Hsp90α represent alternative therapeutic targets.
Similar approaches targeting the extracellular Hsp90α in both tissue repair and cancer progression have been previously explored and results of those studies are consistent. Tsen et al.20 showed that inhibition of extracellular Hsp90α signalling delays wound healing. Tsutsumi et al.39 used a membrane impermeable 17-AAG inhibitors to block the action of extracellular Hsp90 and reported the requirement for extracellular Hsp90 in tumour cell motility and invasion. Stellas et al.40 reported that monoclonal antibody 4C5 against Hsp90α blocks the interaction of extracellular Hsp90 with MMP2 and MMP9 and inhibits breast cancer cell deposits in nude mice. Our newly generated monoclonal antibody, IG6-D7, which selectively targets the dual lysine region in secreted Hsp90α represents a viable anticancer agent of this kind. Nonetheless, continued understanding of the mechanisms that control Hsp90α secretion and function within the tumour microenvironment will guide the design of more effective and less toxic anticancer therapeutics.41
Materials and methods
Cell lines and commercial antibodies
MDA-MB-231 and the control (untransformed) mammary epithelial cell line, HBL-100, were kindly provided by Dr Michael Press (University of Southern California, Los Angeles, CA, USA). Both cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% foetal bovine serum. Before experiments, the cells were deprived of serum, washed in phosphate-buffered saline and incubated in serum-free medium for 16 h. Anti-Hsp90α antibody was from Calbiochem (Darmstadt, Germany). Anti-Hsp90β antibodies were from StressMarq Biosciences Inc. (Victoria, British Columbia, Canada). Anti-phospho-Akt (S-473), anti-phospho-Akt (T308), anti-phospho-ERK and anti-phospho-p38 antibodies were from Cell Signalling Technology Inc. (Danvers, MA, USA). Anti-phospho (T246)-PRAS40 was from R&D Systems (Minneapolis, MN, USA).
Lentiviral systems for up- or downregulation of target genes
The pRRLsinh-CMV system was used to exogenously overexpress genes. The pHR-CMV-puro RNAi delivery system was used to deliver short hairpin RNA against target genes. The detailed protocols were as previously described.2, 20, 33
Production of neutralizing monoclonal antibodies 1G6-D7 and 5C4-D4
The antigen preparation, immunization, screening, antibody purification and antibody epitope mapping are as described in detail (see Supplementary Methods).
CRISPR-Cas9 knockout of Hsp90α and Hsp90β genes
Recombinant Hsp90α and Hsp90β production and purification
Circular dichroism spectroscopy
Preparation of serum-free conditioned medium
The detailed protocols for culturing cells, medium change, incubation period, serum-free conditioned medium collection, medium concentration and analyses were as described previously.2, 10
Colloidal gold cell migration assay
See Supplementary Methods and Li et al.33
Invasion assay
See Supplementary Methods and Sahu et al.2
Tumour assays in mice
Statistical analyses
Change history
04 April 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41388-024-03017-0
References
Young JC, Moarefi I, Hartl FU . Hsp90: a specialized but essential protein-folding tool. J Cell Biol 2001; 154: 267–273.
Sahu D, Zhao Z, Tsen F, Cheng CF, Park R, Situ AJ et al. A potentially common peptide target in secreted heat shock protein-90α for hypoxia-inducible factor-1α-positive tumours. Mol Biol Cell 2012; 23: 602–613.
Dong H, Zou M, Bhatia A, Jayaprakash P, Hofman F, Yin Q, Chen M, Woodley DT, Li W . Breast cancer MDA-MB-231 cells use secreted heat shock protein-90alpha (Hsp90α) to survive a hostile hypoxic environment. Sci Rep 2016; 6: 20605.
Obermann WM, Sondermann H, Russo AA, Pavletich NP, Hartl FU . In vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis. J Cell Biol 1998; 143: 901–910.
Drysdale MJ, Brough PA, Massey A, Jensen MR, Schoepfer J . Targeting Hsp90 for the treatment of cancer. Curr Opin Drug Discov Devel 2006; 9: 483–495.
Workman P . Altered states: selectively drugging the Hsp90 cancer chaperone. Trends Mol Med 2004; 10: 47–51.
Whitesell L, Lindquist SL . HSP90 and the chaperoning of cancer. Nat Rev Cancer 2005; 5: 761–772.
Neckers L, Workman P . Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res 2012; 18: 64–76.
Cheng CF, Fan J, Fedesco M, Guan S, Li Y, Bandyopadhyay B et al. Transforming growth factor alpha (TGFalpha)-stimulated secretion of HSP90alpha: using the receptor LRP-1/CD91 to promote human skin cell migration against a TGFbeta-rich environment during wound healing. Mol Cell Biol 2008; 28: 3344–3358.
Eustace BK, Sakurai T, Stewart JK, Yimlamai D, Unger C, Zehetmeier C et al. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat Cell Biol 6 2004. 507–514.
Li W, Sahu D, Tsen F . Secreted heat shock protein-90 (Hsp90) in wound healing and cancer. Biochim Biophys Acta 2012; 1823: 730–741.
Semenza GL . Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003; 3: 721–732.
Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z . Induction of heat shock proteins in B-cell exosomes. J Cell Sci 2005; 118: 3631–3638.
Yu X, Harris SL, Levine AJ . The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res 2006; 66: 4795–4801.
McCready J, Sims JD, Chan D, Jay DG . Secretion of extracellular hsp90alpha via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer 2010; 10: 294.
Tsutsumi S, Neckers L . Extracellular heat shock protein 90: a role for a molecular chaperone in cell motility and cancer metastasis. Cancer Sci 2007; 98: 1536–1539.
Sidera K, Gaitanou M, Stellas D, Matsas R, Patsavoudi E . A critical role for HSP90 in cancer cell invasion involves interaction with the extracellular domain of HER-2. J Biol Chem 2008; 283: 2031–2041.
El Hamidieh A, Grammatikakis N, Patsavoudi E . Cell surface Cdc37 participates in extracellular HSP90 mediated cancer cell invasion. PLoS One 2012; 7: e42722.
Nolan KD, Franco OE, Hance MW, Hayward SW, Isaacs JS . Tumor-secreted Hsp90 subverts polycomb function to drive prostate tumor growth and invasion. J Biol Chem 2015; 290: 8271–8282.
Tsen F, Bhatia A, O'Brien K, Cheng CF, Chen M, Hay N et al. Extracellular heat shock protein 90 signals through subdomain II and the NPVY motif of LRP-1 receptor to Akt1 and Akt2: a circuit essential for promoting skin cell migration in vitro and wound healing in vivo. Mol Cell Biol 2013; 33: 4947–4959.
Zagouri F, Sergentanis TN, Provatopoulou X, Kalogera E, Chrysikos D, Lymperi M et al. Serum levels of Hsp90 in the continuum of breast ductal and lobular lesions. In Vivo 2011; 25: 669–672.
Shi Y, Liu X, Lou J, Han X, Zhang L, Wang Q et al. Plasma levels of heat shock protein 90alpha associated with lung cancer development and treatment responses. Clin Cancer Res 2014; 20: 6016–6022.
Cailleau R, Young R, Olive M, Reeves WJ Jr . Breast tumour cell lines from pleural effusions. J Natl Cancer Inst 1974; 53: 661–674.
Chen JS, Hsu YM, Chen CC, Chen LL, Lee CC, Huang TS . Secreted heat shock protein 90alpha induces colorectal cancer cell invasion through CD91/LRP-1 and NF-kappaB-mediated integrin alphaV expression. J Biol Chem 2010; 285: 25458–25466.
Devarakonda CV, Kita D, Phoenix KN, Claffey KP . Patient-derived heavy chain antibody targets cell surface HSP90 on breast tumours. BMC Cancer 2015; 5: 614.
Wang X, Song X, Zhuo W, Fu Y, Shi H, Liang Y, Tong M, Chang G, Luo. Y . The regulatory mechanism of Hsp90alpha secretion and its function in tumour malignancy. Proc Natl Acad Sci USA 2009; 106: 21288–21293.
Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE et al. RNA-guided human genome engineering via Cas9. Science 2013; 339: 823–826.
Voss AK, Thomas T, Gruss P . Mice lacking HSP90beta fail to develop a placental labyrinth. Development 2000; 127: 1–11.
Grad I, Cederroth CR, Walicki J, Grey C, Barluenga S, Winssinger N et al. The molecular chaperone Hsp90α is required for meiotic progression of spermatocytes beyond pachytene in the mouse. PLoS One 2010; 5: e15770.
Imai T, Kato Y, Kajiwara C, Mizukami S, Ishige I, Ichiyanagi T et al. Heat shock protein 90 (HSP90) contributes to cytosolic translocation of extracellular antigen for cross-presentation by dendritic cells. Proc Natl Acad Sci USA 2011; 108: 16363–16368.
Albrecht-Buehler G . The phagokinetic tracks of 3T3 cells. Cell 1977; 11: 395–404.
Woodley DT, Bachmann PM, O'Keefe EJ . Laminin inhibits human keratinocyte migration. J Cell Physiol 1988; 136: 140–146.
Li W, Fan J, Chen M, Guan S, Sawcer D, Bokoch GM et al. Mechanism of human dermal fibroblast migration driven by type I collagen and platelet-derived growth factor-BB. Mol Biol Cell 2004; 15: 294–309.
Cheng CF, Sahu D, Tsen F, Zhao Z, Fan J, Kim R et al. A fragment of secreted Hsp90alpha carries properties that enable it to accelerate effectively both acute and diabetic wound healing in mice. J Clin Invest 2011; 121: 4348–4361.
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD et al. Genes that mediate breast cancer metastasis to lung. Nature 2005; 436: 518–524.
Ye Xl, Li Y, Stawicki S, Couto S, Eastham-Anderson J, Kallop D et al. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene 2010; 29: 5254–5264.
Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci USA 2013; 110: 11103–11108.
Liu R, Li X, Gao W, Zhou Y, Wey S, Mitra SK et al. Monoclonal antibody against cell surface GRP78 as a novel agent in suppressing PI3K/AKT signalling, tumour growth, and metastasis. Clin Cancer Res. 2013; 19: 6802–6811.
Tsutsumi S, Scroggins B, Koga F, Lee MJ, Trepel J, Felts S et al. Neckers A small molecule cell-impermeant Hsp90 antagonist inhibits tumor cell motility and invasion. Oncogene 2008; 27: 2478–2487.
Stellas D, El Hamidieh A, Patsavoudi E . Monoclonal antibody 4C5 prevents activation of MMP2 and MMP9 by disrupting their interaction with extracellular HSP90 and inhibits formation of metastatic breast cancer cell deposits. BMC Cell Biol 2010; 11: 51.
McCready J, Wong DS, Burlison JA, Ying W, Jay D . An impermeant ganetespib analog inhibits extracellular Hsp90-mediated cancer cell migration that involves lysyl oxidase 2-like protein. Cancers 2014; 6: 1031–1046.
Acknowledgements
This work is supported by NIH grants GM066193, GM067100 and AR059853 (to WL), AR33625 (MC and DTW) and VA Merit Award I01 BX002028 (to DTW). The project described was also supported in part by award number P30CA014089 from the National Cancer Institute (to USC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Zou, M., Bhatia, A., Dong, H. et al. Evolutionarily conserved dual lysine motif determines the non-chaperone function of secreted Hsp90alpha in tumour progression. Oncogene 36, 2160–2171 (2017). https://doi.org/10.1038/onc.2016.375
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.375
This article is cited by
-
Identification of immunogenic cell death-related genes involved in Alzheimer’s disease
Scientific Reports (2024)
-
Structural and functional complexity of HSP90 in cellular homeostasis and disease
Nature Reviews Molecular Cell Biology (2023)
-
Co-targeting HSP90 alpha and CDK7 overcomes resistance against HSP90 inhibitors in BCR-ABL1+ leukemia cells
Cell Death & Disease (2023)
-
LRP-1 receptor combines EGFR signalling and eHsp90α autocrine to support constitutive breast cancer cell motility in absence of blood supply
Scientific Reports (2022)
-
Heat shock protein-90alpha (Hsp90α) stabilizes hypoxia-inducible factor-1α (HIF-1α) in support of spermatogenesis and tumorigenesis
Cancer Gene Therapy (2021)