Abstract
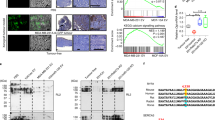
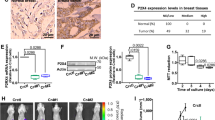
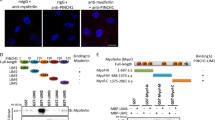
Myoferlin is a multiple C2-domain-containing protein that regulates membrane repair, tyrosine kinase receptor function and endocytosis in myoblasts and endothelial cells. Recently it has been reported as overexpressed in several cancers and shown to contribute to proliferation, migration and invasion of cancer cells. We have previously demonstrated that myoferlin regulates epidermal growth factor receptor activity in breast cancer. In the current study, we report a consistent overexpression of myoferlin in triple-negative breast cancer cells (TNBC) over cells originating from other breast cancer subtypes. Using a combination of proteomics, metabolomics and electron microscopy, we demonstrate that myoferlin depletion results in marked alteration of endosomal system and metabolism. Mechanistically, myoferlin depletion caused impaired vesicle traffic that led to a misbalance of saturated/unsaturated fatty acids. This provoked mitochondrial dysfunction in TNBC cells. As a consequence of the major metabolic stress, TNBC cells rapidly triggered AMP activated protein kinase-mediated metabolic reprogramming to glycolysis. This reduced their ability to balance between oxidative phosphorylation and glycolysis, rendering TNBC cells metabolically inflexible, and more sensitive to metabolic drug targeting in vitro. In line with this, our in vivo findings demonstrated a significantly reduced capacity of myoferlin-deficient TNBC cells to metastasise to lungs. The significance of this observation was further supported by clinical data, showing that TNBC patients whose tumors overexpress myoferlin have worst distant metastasis-free and overall survivals. This novel insight into myoferlin function establishes an important link between vesicle traffic, cancer metabolism and progression, offering new diagnostic and therapeutic concepts to develop treatments for TNBC patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008; 26: 1275–1281.
Mayer IA, Abramson VG, Lehmann BD, Pietenpol JA . New strategies for triple-negative breast cancer—deciphering the heterogeneity. Clin Cancer Res 2014; 20: 782–790.
O'Toole SA, Beith JM, Millar EK, West R, McLean A, Cazet A et al. Therapeutic targets in triple negative breast cancer. J Clin Pathol 2013; 66: 530–542.
Vander Heiden MG . Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov 2011; 10: 671–684.
Warburg O . On the origin of cancer cells. Science 1956; 123: 309–314.
Jose C, Bellance N, Rossignol R . Choosing between glycolysis and oxidative phosphorylation: a tumor's dilemma? Biochim Biophys Acta 2011; 1807: 552–561.
Zheng J . Energy metabolism of cancer: glycolysis versus oxidative phosphorylation (Review). Oncol Lett 2012; 4: 1151–1157.
Griguer CE, Oliva CR, Gillespie GY . Glucose metabolism heterogeneity in human and mouse malignant glioma cell lines. J Neurooncol 2005; 74: 123–133.
Miccheli A, Tomassini A, Puccetti C, Valerio M, Peluso G, Tuccillo F et al. Metabolic profiling by 13C-NMR spectroscopy: [1,2-13C2]glucose reveals a heterogeneous metabolism in human leukemia T cells. Biochimie 2006; 88: 437–448.
Chen JL, Lucas JE, Schroeder T, Mori S, Wu J, Nevins J et al. The genomic analysis of lactic acidosis and acidosis response in human cancers. PLoS Genet 2008; 4: e1000293.
Haq R, Shoag J, Andreu-Perez P, Yokoyama S, Edelman H, Rowe GC et al. Oncogenic BRAF regulates oxidative metabolism via PGC1alpha and MITF. Cancer Cell 2013; 23: 302–315.
Sorkin A, von Zastrow M . Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol 2009; 10: 609–622.
Goldstein JL, Brown MS . The LDL receptor. Arterioscler Thromb Vasc Biol 2009; 29: 431–438.
Aisen P, Enns C, Wessling-Resnick M . Chemistry and biology of eukaryotic iron metabolism. Int J Biochem Cell Biol 2001; 33: 940–959.
Jovic M, Sharma M, Rahajeng J, Caplan S . The early endosome: a busy sorting station for proteins at the crossroads. Histol Histopathol 2010; 25: 99–112.
Antonescu CN, McGraw TE, Klip A . Reciprocal regulation of endocytosis and metabolism. Cold Spring Harb Perspect Biol 2014; 6: a016964.
Rambold AS, Cohen S, Lippincott-Schwartz J . Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev Cell 2015; 32: 678–692.
Unger RH, Clark GO, Scherer PE, Orci L . Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta 2010; 1801: 209–214.
Li R, Ackerman WEt, Mihai C, Volakis LI, Ghadiali S, Kniss DA . Myoferlin depletion in breast cancer cells promotes mesenchymal to epithelial shape change and stalls invasion. PLoS One 2012; 7: e39766.
Turtoi A, Blomme A, Bellahcene A, Gilles C, Hennequiere V, Peixoto P et al. Myoferlin is a key regulator of EGFR activity in breast cancer. Cancer Res 2013; 73: 5438–5448.
Volakis LI, Li R, Ackerman WEt, Mihai C, Bechel M, Summerfield TL et al. Loss of myoferlin redirects breast cancer cell motility towards collective migration. PLoS One 2014; 9: e86110.
Sachen KL, Strohman MJ, Singletary J, Alizadeh AA, Kattah NH, Lossos C et al. Self-antigen recognition by follicular lymphoma B-cell receptors. Blood 2012; 120: 4182–4190.
Turtoi A, Musmeci D, Wang Y, Dumont B, Somja J, Bevilacqua G et al. Identification of novel accessible proteins bearing diagnostic and therapeutic potential in human pancreatic ductal adenocarcinoma. J Proteome Res 2011; 10: 4302–4313.
Wang WS, Liu XH, Liu LX, Lou WH, Jin DY, Yang PY et al. iTRAQ-based quantitative proteomics reveals myoferlin as a novel prognostic predictor in pancreatic adenocarcinoma. J Proteomics 2013; 91: 453–465.
Fahmy K, Gonzalez A, Arafa M, Peixoto P, Bellahcene A, Turtoi A et al. Myoferlin plays a key role in VEGFA secretion and impacts tumor-associated angiogenesis in human pancreas cancer. Int J Cancer 2016; 138: 652–663.
Leung C, Yu C, Lin MI, Tognon C, Bernatchez P . Expression of myoferlin in human and murine carcinoma tumors: role in membrane repair, cell proliferation, and tumorigenesis. Am J Pathol 2013; 182: 1900–1909.
Doherty KR, Demonbreun AR, Wallace GQ, Cave A, Posey AD, Heretis K et al. The endocytic recycling protein EHD2 interacts with myoferlin to regulate myoblast fusion. J Biol Chem 2008; 283: 20252–20260.
Bernatchez PN, Sharma A, Kodaman P, Sessa WC . Myoferlin is critical for endocytosis in endothelial cells. Am J Physiol Cell Physiol 2009; 297: C484–C492.
Bernatchez PN, Acevedo L, Fernandez-Hernando C, Murata T, Chalouni C, Kim J et al. Myoferlin regulates vascular endothelial growth factor receptor-2 stability and function. J Biol Chem 2007; 282: 30745–30753.
Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006; 10: 515–527.
Kiss AL . Caveolae and the regulation of endocytosis. Adv Exp Med Biol 2012; 729: 14–28.
Penzo D, Tagliapietra C, Colonna R, Petronilli V, Bernardi P . Effects of fatty acids on mitochondria: implications for cell death. Biochim Biophys Acta 2002; 1555: 160–165.
Doherty KR, Cave A, Davis DB, Delmonte AJ, Posey A, Earley JU et al. Normal myoblast fusion requires myoferlin. Development 2005; 132: 5565–5575.
Pilch PF, Liu L . Fat caves: caveolae, lipid trafficking and lipid metabolism in adipocytes. Trends Endocrinol Metab 2011; 22: 318–324.
Hullin-Matsuda F, Taguchi T, Greimel P, Kobayashi T . Lipid compartmentalization in the endosome system. Semin Cell Dev Biol 2014; 31: 48–56.
Pavlova NN, Thompson CB . The emerging hallmarks of cancer metabolism. Cell Metab 2016; 23: 27–47.
LeBleu VS, O'Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC et al. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol 2014; 16: 992–1003 1-15.
Porporato PE, Payen VL, Perez-Escuredo J, De Saedeleer CJ, Danhier P, Copetti T et al. A mitochondrial switch promotes tumor metastasis. Cell Rep 2014; 8: 754–766.
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB . The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 2008; 7: 11–20.
Ward PS, Thompson CB . Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 2012; 21: 297–308.
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG . Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010; 8: e1000412.
Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 2015; 21: 263–269.
Matheus N, Hansen S, Rozet E, Peixoto P, Maquoi E, Lambert V et al. An easy, convenient cell and tissue extraction protocol for nuclear magnetic resonance metabolomics. Phytochem Anal 2014; 25: 342–349.
Gyorffy B, Surowiak P, Budczies J, Lanczky A . Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS One 2013; 8: e82241.
Acknowledgements
We acknowledge the experimental support of Dr Chantal Humblet and Mrs Alice Marquet (GIGA-histology platform, ULg), Dr Sandra Ormenese (GIGA-imaging platform, ULg), Mr Vincent Hennequière and Mrs Naima Maloujahmoum (Metastasis Research Laboratory) for experimental support. We are also thankful to the institutional Biobank of the University Hospital Liege for providing patient material. Mr Mathieu Roch (CMMI) is thanked for his help in tumor volume measurements on MR images. We are grateful to Mrs Marie-Aline Laute and Mr Nicolas Passon, the Cyclotron team (Erasme Hospital, Brussels, Belgium) and Dr. Bolag Altan (Gunma University) for technical assistance. The results shown in this work are in part based upon data generated by the TCGA Research Network: http://cancergenome.nih.gov/. This work was supported with grants from the University of Liège (Concerted Research Action Program (IDEA project)), National Fund for Scientific Research (FNRS), Fonds Erasme, Convention de Recherche Association Vinçotte Nuclear—AVN and Gunma University (GIAR Research Program for Omics-Based Medical Science). Andrei Turtoi and Arnaud Blomme are post-doctoral research fellows (FNRS/Televie), Akeila Bellahcène and Pascal de Tullio are senior research associates (FNRS). The Center for Microscopy and Molecular Imaging (CMMI) as well as Gilles Doumont are supported by the European Regional Development Fund and Wallonia (FEDER). No funding bodies had any role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Blomme, A., Costanza, B., de Tullio, P. et al. Myoferlin regulates cellular lipid metabolism and promotes metastases in triple-negative breast cancer. Oncogene 36, 2116–2130 (2017). https://doi.org/10.1038/onc.2016.369
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.369
This article is cited by
-
Identification of myoferlin as a mitochondria-associated membranes component required for calcium signaling in PDAC cell lines
Cell Communication and Signaling (2024)
-
TC2N inhibits distant metastasis and stemness of breast cancer via blocking fatty acid synthesis
Journal of Translational Medicine (2024)
-
Therapeutic protein PAK restrains the progression of triple negative breast cancer through degrading SREBP-1 mRNA
Breast Cancer Research (2023)
-
TMEM196 inhibits lung cancer metastasis by regulating the Wnt/β-catenin signaling pathway
Journal of Cancer Research and Clinical Oncology (2023)
-
HPV infection alters vaginal microbiome through down-regulating host mucosal innate peptides used by Lactobacilli as amino acid sources
Nature Communications (2022)