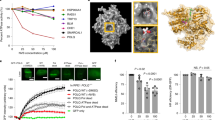
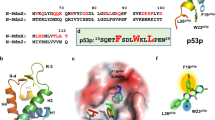
Abstract
Hydrocarbon-stapled peptides that display key residues of the p53 transactivation domain have emerged as bona fide clinical candidates for reactivating the tumor suppression function of p53 in cancer by dual targeting of the negative regulators HDM2 and HDMX. A recent study questioned the mechanistic specificity of such stapled peptides based on interrogating their capacity to disrupt p53/HDM2 and p53/HDMX complexes in living cells using a new recombinase enhanced bimolecular luciferase complementation platform (ReBiL). Here, we directly evaluate the cellular uptake, intracellular targeting selectivity and p53-dependent cytotoxicity of the clinical prototype ATSP-7041. We find that under standard serum-containing tissue culture conditions, ATSP-7041 achieves intracellular access without membrane disruption, dose-dependently dissociates both p53/HDM2 and p53/HDMX complexes but not an unrelated protein complex in long-term ReBiL experiments, and is selectively cytotoxic to cancer cells bearing wild-type p53 by inducing a surge in p53 protein level. These studies underscore the importance of a thorough stepwise approach, including consideration of the time-dependence of cellular uptake and intracellular distribution, in evaluating and advancing stapled peptides for clinical translation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ et al. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science 1996; 274: 948–953.
Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science 1997; 275: 983–986.
Wade M, Li YC, Wahl GM . MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer 2013; 13: 83–96.
Vousden KH, Lane DP . p53 in health and disease. Nat Rev Mol Cell Biol 2007; 8: 275–283.
Honda R, Tanaka H, Yasuda H . Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett 1997; 420: 25–27.
Shvarts A, Steegenga WT, Riteco N, van Laar T, Dekker P, Bazuine M et al. MDMX: a novel p53-binding protein with some functional properties of MDM2. EMBO J 1996; 15: 5349–5357.
Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z et al. in vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004; 303: 844–848.
Bernal F, Wade M, Godes M, Davis TN, Whitehead DG, Kung AL et al. A stapled p53 helix overcomes HDMX-mediated suppression of p53. Cancer Cell 2010; 18: 411–422.
Bernal F, Tyler AF, Korsmeyer SJ, Walensky LD, Verdine GL . Reactivation of the p53 tumor suppressor pathway by a stapled p53 peptide. J Am Chem Soc 2007; 129: 2456–2457.
Chang YS, Graves B, Guerlavais V, Tovar C, Packman K, To KH et al. Stapled alpha-helical peptide drug development: a potent dual inhibitor of MDM2 and MDMX for p53-dependent cancer therapy. Proc Natl Acad Sci USA 2013; 110: E3445–E3454.
Tan BX, Brown CJ, Ferrer FJ, Yuen TY, Quah ST, Chan BH et al. Assessing the efficacy of Mdm2/Mdm4-inhibiting stapled peptides using cellular thermal shift assays. Sci Rep 2015; 5: 12116.
Brown CJ, Quah ST, Jong J, Goh AM, Chiam PC, Khoo KH et al. Stapled peptides with improved potency and specificity that activate p53. ACS Chem Biol 2013; 8: 506–512.
Li YC, Rodewald LW, Hoppmann C, Wong ET, Lebreton S, Safar P et al. A versatile platform to analyze low-affinity and transient protein-protein interactions in living cells in real time. Cell Rep 2014; 9: 1946–1958.
Bird GH, Bernal F, Pitter K, Walensky LD . Synthesis and biophysical characterization of stabilized alpha-helices of BCL-2 domains. Methods Enzymol 2008; 446: 369–386.
Bird GH, Crannell WC, Walensky LD . Chemical synthesis of hydrocarbon-stapled peptides for protein interaction research and therapeutic targeting. Curr Protoc Chem Biol 2011; 3: 99–117.
Walensky LD, Bird GH . Hydrocarbon-stapled peptides: principles, practice, and progress. J Med Chem 2014; 57: 6275–6288.
Edwards AL, Wachter F, Lammert M, Huhn AJ, Luccarelli J, Bird GH et al. Cellular uptake and ultrastructural localization underlie the pro-apoptotic activity of a hydrocarbon-stapled BIM BH3 peptide. ACS Chem Biol 2015; 10: 2149–2157.
Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD et al. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science 2004; 305: 1466–1470.
Baker SJ, Fearon ER, Nigro JM, Hamilton SR, Preisinger AC, Jessup JM et al. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science 1989; 244: 217–221.
Moll UM, LaQuaglia M, Benard J, Riou G . Wild-type p53 protein undergoes cytoplasmic sequestration in undifferentiated neuroblastomas but not in differentiated tumors. Proc Natl Acad Sci USA 1995; 92: 4407–4411.
Nigro JM, Baker SJ, Preisinger AC, Jessup JM, Hostetter R, Cleary K et al. Mutations in the p53 gene occur in diverse human tumour types. Nature 1989; 342: 705–708.
Bird GH, Gavathiotis E, LaBelle JL, Katz SG, Walensky LD . Distinct BimBH3 (BimSAHB) stapled peptides for structural and cellular studies. ACS Chem Biol 2014; 9: 831–837.
LaBelle JL, Katz SG, Bird GH, Gavathiotis E, Stewart ML, Lawrence C et al. A stapled BIM peptide overcomes apoptotic resistance in hematologic cancers. J Clin Invest 2012; 122: 2018–2031.
Okamoto T, Zobel K, Fedorova A, Quan C, Yang H, Fairbrother WJ et al. Stabilizing the pro-apoptotic BimBH3 helix (BimSAHB) does not necessarily enhance affinity or biological activity. ACS Chem Biol 2013; 8: 297–302.
Li M, Brooks CL, Wu-Baer F, Chen D, Baer R, Gu W . Mono- versus polyubiquitination: differential control of p53 fate by Mdm2. Science 2003; 302: 1972–1975.
Acknowledgements
We thank E Smith for graphical assistance, Lisa Cameron for assistance with confocal microscopy, and Alexandra Zoraian and Katherine Franklin for technical support. We appreciate the generosity of Drs Y Li and G Wahl for providing the ReBiL cells for this study, hosting FW in their laboratory to conduct confirmatory experiments and for helpful feedback on the manuscript. This work was supported by a Hyundai Hope on Wheels Quantum Award, Alex’s Lemonade Stand Reach Grant, the Todd J Schwartz Memorial Fund, and Leukemia and Lymphoma SCOR and Scholar Awards to LDW. FW is supported by an Alexander von Humboldt Foundation Feodor Lynen Fellowship, AMM by NIH training grant T32GM007753, RM by Doctoral Foreign Studies Award DFS-134963 from the Canadian Institutes of Health Research, and AZ and KF by the William Lawrence and Blanche Hughes Foundation and a generous gift from Jim and Lisa LaTorre, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
LDW is a scientific advisory board member and consultant for Aileron Therapeutics.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Wachter, F., Morgan, A., Godes, M. et al. Mechanistic validation of a clinical lead stapled peptide that reactivates p53 by dual HDM2 and HDMX targeting. Oncogene 36, 2184–2190 (2017). https://doi.org/10.1038/onc.2016.361
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.361