Abstract
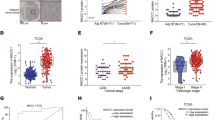
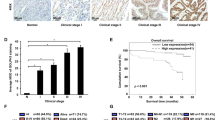
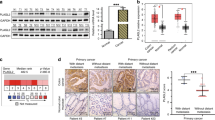
We previously demonstrated that fermitin family member 1 (FERMT1) was significantly overexpressed in colon cancer (CC) and associated with poor metastasis-free survival. This study aimed to investigate the precise role of FERMT1 in CC metastasis and the mechanism by which FERMT1 is involved in the epithelial–mesenchymal transition (EMT). Correlations between FERMT1 and EMT markers (E-cadherin, Slug, N-cadherin and β-catenin) were examined via immunohistochemistry in a cohort of CC tissues and adjacent normal colon mucosae. A series of in vitro and in vivo assays were performed to elucidate the function of FERMT1 in CC metastasis and underlying mechanisms. The upregulated expression of FERMT1 in CC tissues correlated positively with that of Slug, N-cadherin and β-catenin, but correlated inversely with E-cadherin expression. Altered FERMT1 expression led to marked changes in the proliferation, migration, invasion and EMT markers of CC cells both in vitro and in vivo. Investigations of underlying mechanisms found that FERMT1 interacted directly with β-catenin and activated the Wnt/β-catenin signaling pathway by decreasing the phosphorylation level of β-catenin, enhancing β-catenin nuclear translocation and increasing the transcriptional activity of β-catenin/TCF/LEF. Activation of the Wnt/β-catenin pathway by CHIR99021 reversed the effect of FERMT1 knockdown, whereas inhibition of the Wnt/β-catenin pathway by XAV939 impaired the effect of FERMT1 overexpression on EMT and cell motility. In conclusion, findings of this study suggest that FERMT1 activates the β-catenin transcriptional activity to promote EMT in CC metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Siegel R, Desantis C, Jemal A . Colorectal cancer statistics, 2014. CA Cancer J Clin 2014; 64: 104–117.
Chaffer CL, Weinberg RA . A perspective on cancer cell metastasis. Science 2011; 331: 1559–1564.
Thiery JP, Acloque H, Huang RY, Nieto MA . Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139: 871–890.
Sleeman JP, Thiery JP . SnapShot: the epithelial-mesenchymal transition. Cell 2011; 145: 162.e161.
Larue L, Bellacosa A . Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3' kinase/AKT pathways. Oncogene 2005; 24: 7443–7454.
Thiery JP, Sleeman JP . Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 2006; 7: 131–142.
Nawshad A, Lagamba D, Polad A, Hay ED . Transforming growth factor-beta signaling during epithelial-mesenchymal transformation: implications for embryogenesis and tumor metastasis. Cells Tissues Organs 2005; 179: 11–23.
Kalluri R, Weinberg RA . The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119: 1420–1428.
White BD, Chien AJ, Dawson DW . Dysregulation of Wnt/beta-catenin signaling in gastrointestinal cancers. Gastroenterology 2012; 142: 219–232.
Valenta T, Hausmann G, Basler K . The many faces and functions of beta-catenin. EMBO J 2012; 31: 2714–2736.
Gonzalez DM, Medici D . Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal 2014; 7: re8.
Niehrs C . The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol 2012; 13: 767–779.
Fodde R, Brabletz T . Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol 2007; 19: 150–158.
Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P et al. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature 2008; 452: 650–653.
Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP et al. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell 2012; 149: 1245–1256.
Thiery JP . Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2: 442–454.
Fan J, Yan D, Teng M, Tang H, Zhou C, Wang X et al. Digital transcript profile analysis with aRNA-LongSAGE validates FERMT1 as a potential novel prognostic marker for colon cancer. Clin Cancer Res 2011; 17: 2908–2918.
Siegel DH, Ashton GH, Penagos HG, Lee JV, Feiler HS, Wilhelmsen KC et al. Loss of kindlin-1, a human homolog of the Caenorhabditis elegans actin-extracellular-matrix linker protein UNC-112, causes Kindler syndrome. Am J Hum Genet 2003; 73: 174–187.
Larjava H, Plow EF, Wu C . Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep 2008; 9: 1203–1208.
Ussar S, Moser M, Widmaier M, Rognoni E, Harrer C, Genzel-Boroviczeny O et al. Loss of kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. PLoS Genet 2008; 4: e1000289.
Sin S, Bonin F, Petit V, Meseure D, Lallemand F, Bieche I et al. Role of the focal adhesion protein kindlin-1 in breast cancer growth and lung metastasis. J Natl Cancer Inst 2011; 103: 1323–1337.
Mahawithitwong P, Ohuchida K, Ikenaga N, Fujita H, Zhao M, Kozono S et al. Kindlin-1 expression is involved in migration and invasion of pancreatic cancer. Int J Oncol 2013; 42: 1360–1366.
Ma HX, Shu QH, Pan JJ, Liu D, Xu GL, Li JS et al. Expression of kindlin-1 in human hepatocellular carcinoma and its prognostic significance. Tumour Biol 2015; 36: 4235–4241.
Kiriyama K, Hirohashi Y, Torigoe T, Kubo T, Tamura Y, Kanaseki T et al. Expression and function of FERMT genes in colon carcinoma cells. Anticancer Res 2013; 33: 167–173.
Willert K, Nusse R . Beta-catenin: a key mediator of Wnt signaling. Curr Opin Genet Dev 1998; 8: 95–102.
Fagotto F . Looking beyond the Wnt pathway for the deep nature of beta-catenin. EMBO Rep 2013; 14: 422–433.
Wu D, Pan W . GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci 2010; 35: 161–168.
Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA . Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995; 378: 785–789.
Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 2009; 461: 614–620.
Yu H, Ye W, Wu J, Meng X, Liu RY, Ying X et al. Overexpression of sirt7 exhibits oncogenic property and serves as a prognostic factor in colorectal cancer. Clin Cancer Res 2014; 20: 3434–3445.
Eccles SA, Welch DR . Metastasis: recent discoveries and novel treatment strategies. Lancet 2007; 369: 1742–1757.
Gupta GP, Massague J . Cancer metastasis: building a framework. Cell 2006; 127: 679–695.
Puisieux A, Brabletz T, Caramel J . Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol 2014; 16: 488–494.
Loboda A, Nebozhyn MV, Watters JW, Buser CA, Shaw PM, Huang PS et al. EMT is the dominant program in human colon cancer. BMC Med Genomics 2011; 4: 9.
Herz C, Aumailley M, Schulte C, Schlotzer-Schrehardt U, Bruckner-Tuderman L, Has C . Kindlin-1 is a phosphoprotein involved in regulation of polarity, proliferation, and motility of epidermal keratinocytes. J Biol Chem 2006; 281: 36082–36090.
Has C, Ludwig RJ, Herz C, Kern JS, Ussar S, Ochsendorf FR et al. C-terminally truncated kindlin-1 leads to abnormal adhesion and migration of keratinocytes. Br J Dermatol 2008; 159: 1192–1196.
Lai-Cheong JE, Parsons M, Tanaka A, Ussar S, South AP, Gomathy S et al. Loss-of-function FERMT1 mutations in kindler syndrome implicate a role for fermitin family homolog-1 in integrin activation. Am J Pathol 2009; 175: 1431–1441.
Weng W, Feng J, Qin H, Ma Y . Molecular therapy of colorectal cancer: progress and future directions. Int J Cancer 2015; 136: 493–502.
Rognoni E, Widmaier M, Jakobson M, Ruppert R, Ussar S, Katsougkri D et al. Kindlin-1 controls Wnt and TGF-beta availability to regulate cutaneous stem cell proliferation. Nat Med 2014; 20: 350–359.
Kern JS, Herz C, Haan E, Moore D, Nottelmann S, von Lilien T et al. Chronic colitis due to an epithelial barrier defect: the role of kindlin-1 isoforms. J Pathol 2007; 213: 462–470.
Walther A, Johnstone E, Swanton C, Midgley R, Tomlinson I, Kerr D . Genetic prognostic and predictive markers in colorectal cancer. Nat Rev Cancer 2009; 9: 489–499.
Yan DW, Li DW, Yang YX, Xia J, Wang XL, Zhou CZ et al. Ubiquitin D is correlated with colon cancer progression and predicts recurrence for stage II-III disease after curative surgery. Brit J Cancer 2010; 103: 961–969.
Thaker PH, Deavers M, Celestino J, Thornton A, Fletcher MS, Landen CN et al. EphA2 expression is associated with aggressive features in ovarian carcinoma. Clin Cancer Res 2004; 10: 5145–5150.
Acknowledgements
This work was supported by National Natural Science Foundation of China (no. 81172328) and Medical Guidance Project of Shanghai Science and Technology Commission (114119a4600) (124119a1700). We thank Jiachen Nan for her generous help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, CC., Cai, DL., Sun, F. et al. FERMT1 mediates epithelial–mesenchymal transition to promote colon cancer metastasis via modulation of β-catenin transcriptional activity. Oncogene 36, 1779–1792 (2017). https://doi.org/10.1038/onc.2016.339
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.339
This article is cited by
-
FERMT1 promotes cell migration and invasion in non-small cell lung cancer via regulating PKP3-mediated activation of p38 MAPK signaling
BMC Cancer (2024)
-
IFIT1 modulates the proliferation, migration and invasion of pancreatic cancer cells via Wnt/β-catenin signaling
Cellular Oncology (2024)
-
IGFBP2 drives epithelial-mesenchymal transition in hepatocellular carcinoma via activating the Wnt/β-catenin pathway
Infectious Agents and Cancer (2023)
-
ERBB3 binding protein 1 promotes the progression of malignant melanoma through activation of the Wnt/ β-catenin signaling pathway
Cancer Cell International (2022)
-
Multiple roles for Bcl-3 in mammary gland branching, stromal collagen invasion, involution and tumor pathology
Breast Cancer Research (2022)