Abstract
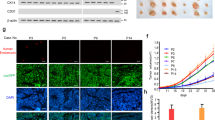
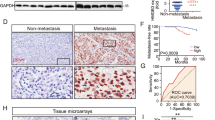
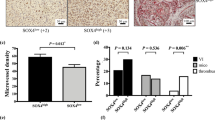
Metastasis of the cervical lymph nodes frequently leads to poor survival of patients with oral squamous cell carcinoma (OSCC). The underlying mechanisms of lymph node metastasis are unclear. Wingless-type MMTV integration site family, member 5B (WNT5B), one component of the WNT signal pathway, was markedly up-regulated in OSCC sublines with high potential of lymphatic metastasis compared to that in OSCC cells with low nodal metastasis. Increased WNT5B mRNA was demonstrated in human OSCC tissues in comparison with adjacent non-tumorous tissues. Interestingly, the high level of WNT5B protein in serum was associated with lymph node metastasis in OSCC patients. Knockdown of WNT5B expression in OSCC sublines did not affect tumour growth but impaired lymph node metastasis and tumour lymphangiogenesis of orthotopic transplantation. Conditioned medium from WNT5B knockdown cells reduced the tube formation of lymphatic endothelial cells (LECs). In contrast, recombinant WNT5B enhanced the tube formation, permeability and migration of LECs. In LECs stained with phalloidin, the morphology of those treated with recombinant WNT5B changed from flat to spindle-like. Recombinant WNT5B also increased α-smooth muscle actin and inhibited the expression of vascular endothelial-cadherin but retained characteristics of endothelial cells. The results suggest that WNT5B functions in the partial endothelial-mesenchymal transition (EndoMT). Furthermore, WNT5B-induced tube formation was impaired in the LECs following the knockdown of EndoMT-related transcription factor, SNAIL or SLUG. The WNT5B-induced expression of Snail or Slug was abolished by IWR-1-endo and Rac1 inhibitors, which are involved in the WNT/β-catenin and planar cell polarity pathways, respectively. Collectively, the data suggest that WNT5B induces tube formation by regulating the expression of Snail and Slug proteins through activation of canonical and non-canonical WNT signalling pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Abbreviations
- OSCC:
-
oral squamous cell carcinoma
- WNT5B:
-
Wingless-type MMTV integration site family member 5B
- LECs:
-
lymphatic endothelial cells
- ECs:
-
endothelial cells
- EndoMT:
-
endothelial-mesenchymal transition
- VE-cadherin:
-
vascular endothelial (VE)-cadherin
- PCP:
-
planar cell polarity
- JNK:
-
c-jun N-terminal kinase
- VEGFC:
-
vascular endothelial growth factor C
- VEGFD:
-
vascular endothelial growth factor D
- qRT-PCR:
-
quantitative reverse transcription-polymerase chain reaction
- ELISA:
-
enzyme-linked immunosorbent assay.
References
Liao CT, Chang JT, Wang HM, Ng SH, Hsueh C, Lee LY et al. Pretreatment primary tumor SUVmax measured by FDG-PET and pathologic tumor depth predict for poor outcomes in patients with oral cavity squamous cell carcinoma and pathologically positive lymph nodes. Int J Radiat Oncol Biol Phys 2009; 73: 764–771.
Sleeman JP, Thiele W . Tumor metastasis and the lymphatic vasculature. Int J Cancer 2009; 125: 2747–2756.
Dieterich LC, Detmar M . Tumor lymphangiogenesis and new drug development. Adv Drug Deliv Rev 2015; 99: 148–160.
Achen MG, Stacker SA . Molecular control of lymphatic metastasis. Ann N Y Acad Sci 2008; 1131: 225–234.
Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG . Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 2014; 14: 159–172.
Buttler K, Becker J, Pukrop T, Wilting J . Maldevelopment of dermal lymphatics in Wnt5a-knockout-mice. Dev Biol 2013; 381: 365–376.
Nicenboim J, Malkinson G, Lupo T, Asaf L, Sela Y, Mayseless O et al. Lymphatic vessels arise from specialized angioblasts within a venous niche. Nature 2015; 522: 56–61.
Dale TC . Signal transduction by the Wnt family of ligands. Biochem J 1998; 329 (Pt 2): 209–223.
Polakis P . Wnt signaling in cancer. Cold Spring Harb Perspect Biol 2012; 4: a008052.
MacDonald BT, Tamai K, He X . Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 2009; 17: 9–26.
Nusse R, Varmus HE . Wnt genes. Cell 1992; 69: 1073–1087.
Kestler HA, Kuhl M . From individual Wnt pathways towards a Wnt signalling network. Philos Trans R Soc Lond B Biol Sci 2008; 363: 1333–1347.
Macheda ML, Sun WW, Kugathasan K, Hogan BM, Bower NI, Halford MM et al. The Wnt receptor Ryk plays a role in mammalian planar cell polarity signaling. J Biol Chem 2012; 287: 29312–29323.
Andre P, Wang Q, Wang N, Gao B, Schilit A, Halford MM et al. The Wnt coreceptor Ryk regulates Wnt/planar cell polarity by modulating the degradation of the core planar cell polarity component Vangl2. J Biol Chem 2012; 287: 44518–44525.
Green JL, Kuntz SG, Sternberg PW . Ror receptor tyrosine kinases: orphans no more. Trends Cell Biol 2008; 18: 536–544.
Yen YC, Hsiao JR, Jiang SS, Chang JS, Wang SH, Shen YY et al. Insulin-like growth factor-independent insulin-like growth factor binding protein 3 promotes cell migration and lymph node metastasis of oral squamous cell carcinoma cells by requirement of integrin beta1. Oncotarget 2015; 6: 41837–41855.
Takeshita A, Iwai S, Morita Y, Niki-Yonekawa A, Hamada M, Yura Y . Wnt5b promotes the cell motility essential for metastasis of oral squamous cell carcinoma through active Cdc42 and RhoA. Int J Oncol 2014; 44: 59–68.
Yang L, Perez AA, Fujie S, Warden C, Li J, Wang Y et al. Wnt modulates MCL1 to control cell survival in triple negative breast cancer. BMC Cancer 2014; 14: 124.
Dejana E . The role of wnt signaling in physiological and pathological angiogenesis. Circ Res 2010; 107: 943–952.
Masckauchan TN, Agalliu D, Vorontchikhina M, Ahn A, Parmalee NL, Li CM et al. Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2. Mol Biol Cell 2006; 17: 5163–5172.
Goodwin AM, Kitajewski J, D’Amore PA . Wnt1 and Wnt5a affect endothelial proliferation and capillary length; Wnt2 does not. Growth Factors 2007; 25: 25–32.
Cheng CW, Yeh JC, Fan TP, Smith SK, Charnock-Jones DS . Wnt5a-mediated non-canonical Wnt signalling regulates human endothelial cell proliferation and migration. Biochem Biophys Res Commun 2008; 365: 285–290.
Lin F, Wang N, Zhang TC . The role of endothelial-mesenchymal transition in development and pathological process. IUBMB Life 2012; 64: 717–723.
Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R . Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res 2007; 67: 10123–10128.
Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 2007; 13: 952–961.
Kokudo T, Suzuki Y, Yoshimatsu Y, Yamazaki T, Watabe T, Miyazono K . Snail is required for TGFbeta-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells. J Cell Sci 2008; 121: 3317–3324.
Cooley BC, Nevado J, Mellad J, Yang D St, Hilaire C, Negro A et al. TGF-beta signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci Transl Med 2014; 6: 227ra234.
Xu X, Tan X, Tampe B, Sanchez E, Zeisberg M, Zeisberg EM . Snail is a direct target of hypoxia-inducible factor 1alpha (HIF1alpha) in hypoxia-induced endothelial to mesenchymal transition of human coronary endothelial cells. J Biol Chem 2015; 290: 16653–16664.
Kato S, Hayakawa Y, Sakurai H, Saiki I, Yokoyama S . Mesenchymal-transitioned cancer cells instigate the invasion of epithelial cancer cells through secretion of WNT3 and WNT5B. Cancer Sci 2013; 105: 281–289.
Hong YK, Detmar M . Prox1, master regulator of the lymphatic vasculature phenotype. Cell Tissue Res 2003; 314: 85–92.
Miyahara M, Tanuma J, Sugihara K, Semba I . Tumor lymphangiogenesis correlates with lymph node metastasis and clinicopathologic parameters in oral squamous cell carcinoma. Cancer 2007; 110: 1287–1294.
Deraz EM, Kudo Y, Yoshida M, Obayashi M, Tsunematsu T, Tani H et al. MMP-10/stromelysin-2 promotes invasion of head and neck cancer. PLoS One 2011; 6: e25438.
Mangioni S, Vigano P, Lattuada D, Abbiati A, Vignali M, Di Blasio AM . Overexpression of the Wnt5b gene in leiomyoma cells: implications for a role of the Wnt signaling pathway in the uterine benign tumor. J Clin Endocrinol Metab 2005; 90: 5349–5355.
Quail DF, Joyce JA . Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013; 19: 1423–1437.
Tobler NE, Detmar M . Tumor and lymph node lymphangiogenesis – impact on cancer metastasis. J Leukoc Biol 2006; 80: 691–696.
Welch-Reardon KM, Wu N, Hughes CC . A role for partial endothelial-mesenchymal transitions in angiogenesis? Arterioscler Thromb Vasc Biol 2015; 35: 303–308.
Ghiabi P, Jiang J, Pasquier J, Maleki M, Abu-Kaoud N, Halabi N et al. Breast cancer cells promote a notch-dependent mesenchymal phenotype in endothelial cells participating to a pro-tumoral niche. J Transl Med 2015; 13: 27.
Welch-Reardon KM, Ehsan SM, Wang K, Wu N, Newman AC, Romero-Lopez M et al. Angiogenic sprouting is regulated by endothelial cell expression of Slug. J Cell Sci 2014; 127: 2017–2028.
Yang Y, Topol L, Lee H, Wu J . Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development 2003; 130: 1003–1015.
Kanazawa A, Tsukada S, Kamiyama M, Yanagimoto T, Nakajima M, Maeda S . Wnt5b partially inhibits canonical Wnt/beta-catenin signaling pathway and promotes adipogenesis in 3T3-L1 preadipocytes. Biochem Biophys Res Commun 2005; 330: 505–510.
Yen YC, Shiah SG, Chu HC, Hsu YM, Hsiao JR, Chang JY et al. Reciprocal regulation of microRNA-99a and insulin-like growth factor I receptor signaling in oral squamous cell carcinoma cells. Mol Cancer 2014; 13: 6.
Lin ZS, Chu HC, Yen YC, Lewis BC, Chen YW . Kruppel-like factor 4, a tumor suppressor in hepatocellular carcinoma cells reverts epithelial mesenchymal transition by suppressing slug expression. PLoS One 2012; 7: e43593.
Festing MF, Altman DG . Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J 2002; 43: 244–258.
Chen YW, Klimstra DS, Mongeau ME, Tatem JL, Boyartchuk V, Lewis BC . Loss of p53 and Ink4a/Arf cooperate in a cell autonomous fashion to induce metastasis of hepatocellular carcinoma cells. Cancer Res 2007; 67: 7589–7596.
Chen YW, Paliwal S, Draheim K, Grossman SR, Lewis BC . p19Arf inhibits the invasion of hepatocellular carcinoma cells by binding to C-terminal binding protein. Cancer Res 2008; 68: 476–482.
Pan MR, Chang TM, Chang HC, Su JL, Wang HW, Hung WC . Sumoylation of Prox1 controls its ability to induce VEGFR3 expression and lymphatic phenotypes in endothelial cells. J Cell Sci 2009; 122: 3358–3364.
Lee CH, Wong TS, Chan JY, Lu SC, Lin P, Cheng AJ et al. Epigenetic regulation of the X-linked tumour suppressors BEX1 and LDOC1 in oral squamous cell carcinoma. J Pathol 2013; 230: 298–309.
Acknowledgements
The authors thank Dr Wen-Chun Hung (National Institute of Cancer Research, National Health Research Institutes, Tainan, Taiwan) for providing technical support and reagents and Pathology Core Laboratory (National Health Research Institutes, Miaoli, Taiwan) for the scoring of immunohistochemistry staining. RNAi reagents were obtained from the National RNAi Core Facility located at the Institute of Molecular Biology/Genomic Research Center, Academia Sinica, supported by the National Research Program for Genomic Medicine Grants of NSC (NSC 97-3112-B-001-016). We are also grateful to the Tissue Bank, Research Center of Clinical Medicine, National Cheng Kung University Hospital for providing clinical samples. This study was supported by grants MOST 104-2314-B-400-014, NHRI CA-104-PP-04 and MOHW105-TDU-B-212-112015 from Ministry of Science and Technology, National Health Research Institutes and Ministry of Health and Welfare, Taiwan, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, SH., Chang, J., Hsiao, JR. et al. Tumour cell-derived WNT5B modulates in vitro lymphangiogenesis via induction of partial endothelial-mesenchymal transition of lymphatic endothelial cells. Oncogene 36, 1503–1515 (2017). https://doi.org/10.1038/onc.2016.317
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.317
This article is cited by
-
Lymphatic vessel: origin, heterogeneity, biological functions, and therapeutic targets
Signal Transduction and Targeted Therapy (2024)
-
Cancer-associated fibroblast-derived PAI-1 promotes lymphatic metastasis via the induction of EndoMT in lymphatic endothelial cells
Journal of Experimental & Clinical Cancer Research (2023)
-
WNT ligands in non-small cell lung cancer: from pathogenesis to clinical practice
Discover Oncology (2023)
-
CD45− erythroid progenitor cells promote lymph node metastasis in gastric cancer by inducing a hybrid epithelial/mesenchymal state in lymphatic endothelial cells
Gastric Cancer (2023)
-
Exosome-derived miR-142-5p remodels lymphatic vessels and induces IDO to promote immune privilege in the tumour microenvironment
Cell Death & Differentiation (2021)