Abstract
The plasma membrane-associated tyrosine phosphatase PTPRO is frequently transcriptionally repressed in cancers and signifies poor prognosis of breast cancer patients. In this study, deletion of Ptpro in MMTV-Erbb2 transgenic mice dramatically shortened the mammary tumor latency and accelerated tumor growth due to loss of Ptpro within the breast cancer cells but not in surrounding tissue as confirmed by hetero-transplantation studies. Both in vitro and in vivo data demonstrated that the phosphatase activity was required for the inactivation of ERBB2 and its downstream signaling. PTPRO regulated the phosphorylation status of ERBB2 at Y1248. Co-immunoprecipitation and proximity ligation assay (Duolink) indicated that PTPRO directly physically interacted with ERBB2. Moreover, PTPRO phosphatase activity shortened the half-life of ERBB2 by increasing endocytotic degradation. PTPRO reexpression by demethylation treatment using 5-azacytidine reduced the proliferation and colony formation potential in ERBB2-positive breast cancer cells. Taken together, PTPRO inhibited ERBB2-driven breast cancer through dephosphorylation leading to dual effects of ERBB2 signaling suppression and endosomal internalization of ERBB2, Therefore, reexpression of PTPRO may be a potential therapy for ERBB2-overexpressing breast cancer.
Similar content being viewed by others

Introduction
Dysregulation of the epidermal growth factor receptors (EGFRs; that is, type I receptor tyrosine kinases (RTKs): ERBB1 (EGFR), ERBB2 (HER2), ERBB3 and ERBB4) drives the development and progression of a wide range of cancers.1 Recently, transcriptome-wide array-based analyses have been used to classify human breast cancer into four main molecular types: luminal A, luminal B, ERBB2-enriched and basal-like.1 ERBB2-enriched breast cancers with ERBB2 amplification account for approximately a quarter of all breast cancer and is associated with poor prognosis.1, 2, 3, 4 Despite the clinical benefits resulted from ERBB2-targeted therapeutics, a substantial percentage of ERBB2-overexpressing cancer fail to respond or develop secondary resistance to the current targeted treatments.2, 3, 4 Thus, for a complete understanding of ERBB2 functions, it is critical to identify the novel mechanistic control of ERBB2 signaling, which will advance the intervention and diagnosis for ERBB2-positive cancers.
Reversible phosphorylation of a specific tyrosine residue is governed by the balanced action of PTKs and protein tyrosine phosphatases (PTPs). Specifically in ERBB2-overexpressing breast cancer, ERBB2 dimerization initiates phosphorylation on tyrosine residues in the cytoplasmic tail of ERBB2,5, 6 resulting in activation of downstream signaling that drives tumor growth.7 Dysregulation of PTPs has been recognized as an important cause of cancers.8, 9, 10 PTP receptor type O (PTPRO, also known as GLEPP1) is a member of the transmembrane receptor family of PTPs that is phylogenetically on a branch of the tyrosine phosphatome distinct from other PTPs.11, 12, 13, 14, 15, 16, 17 Besides its functions in embryonic development, immune response and neuron differentiation,18, 19 PTPRO has been assumed to act as a putative tumor suppressor in several cancer types.20, 21, 22, 23 We recently presented evidence that the DNA methylation status of PTPRO is a prognostic factor in ERBB2-positive breast cancer.24 However, the inherent role of PTPRO in oncogenesis has not been established in physiologically relevant whole animal models. The current knowledge gaps also include the following: the specific tyrosine residue of ERBB2 that is selectively dephosphorylated by PTPRO is unknown; the mechanism by which PTPRO inhibits ERBB2-driven tumorigenesis remains largely unknown; the potential of PTPRO as a therapeutic target in breast cancer has not been evaluated.
In this study, we investigated these unknown questions, and discovered that the loss of Ptpro resulted in amplified ERBB2 oncogenic signaling, feeding into cancerous phenotypes in genetic models and ERBB2-overexpressing human breast tumors. Meanwhile, we discovered the novel mechanisms responsible for tumor suppression by PTPRO, which involved dephosphorylation leading to not only blockade of ERBB2 signaling but also endocytotic degradation. Further, we revealed the therapeutic potential of reexpression of PTPRO by demethylation treatment.
Results
Ptpro deletion enhanced mammary tumorigenesis in MMTV-Erbb2 transgenic mice
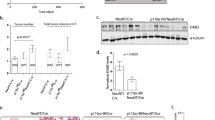
The major knowledge gap about the role of Ptpro in carcinogenesis is the lack of in vivo evidence. To validate the tumor-suppressor role of PTPRO, we examined the influence of Ptpro knockout (Ptpro−/−) on MMTV-Erbb2-driven mammary tumorigenesis in mice. To minimize the variability of tumor formation due to strain difference and heterogeneity of genetic background, we first obtained the Ptpro−/− genotype in a near-pure FVB/N genetic background (99.90% FVB/N) by backcrossing Ptpro−/− mice to FVB/N mice for 10 generations (see the Methods for details). No spontaneous mammary tumors were observed in 30 virgin Ptpro−/− female mice and 32 Ptpro−/− breeding dames with multiple pregnancies after follow-up periods of up to 2 years. This suggested that loss of Ptpro alone might not be sufficient to induce breast tumorigenesis. We investigated the influence of deleting Ptpro on Erbb2-driven mammary gland tumorigenesis by crossing MMTV-Erbb2 mice (100% FVB/N) with Ptpro−/− mice (99.90% FVB/N). We compared breast tumorigenesis in Ptpro−/−/MMTV-Erbb2 with Ptpro+/+/MMTV-Erbb2 mice. In a longitudinal study, palpable mammary tumors were detected between 26 and 49 weeks of age in 35 Ptpro+/+/MMTV-Erbb2 virgin female mice (one mouse was lost soon after genotyping); in contrast, palpable tumors were detected in 36 Ptpro−/−/MMTV-Erbb2 virgin female mice between 17 and 34 weeks of age (Figure 1a). The median time to detection of breast tumors was significantly shorter in Ptpro−/−/MMTV-Erbb2 compared with Ptpro+/+/MMTV-Erbb2 (median: 27 weeks vs 36 weeks, respectively; P<0.001, log rank test). All the mice were killed at 9 weeks after tumor detection. The dissected index tumors in Ptpro−/−/MMTV-Erbb2 mice weighed significantly more than those in Ptpro+/+/MMTV-Erbb2 mice at the same time point after tumor detection (P=0.017; Figure 1b and Supplementary Figure 1A). To compare the rate of tumor progression, we measured the tumor sizes for 9 weeks since detection. Tumor volumes from week 5 to week 9 in the Ptpro−/−/MMTV-Erbb2 group were significantly larger than the corresponding volumes in the Ptpro+/+/MMTV-Erbb2 group (Figure 1c). We also documented the number of tumors at 9 weeks after the first tumor was detected. Together, deletion of Ptpro remarkably accelerated Erbb2-driven mammary tumorigenesis, validating the long suspected tumor-suppressor activity of Ptpro in a whole animal model of breast cancer for the first time.
Ptpro deficiency facilitated MMTV-Erbb2-induced mammary tumorigenesis. (a) Kaplan–Meier plots of tumor-free survival in Ptpro+/+/MMTV-Erbb2 and Ptpro−/−/MMTV-Erbb2 mice. (b) Representative images of tumors from all of the Ptpro−/−/MMTV-Erbb2 mice and littermate control (left). Harvested tumor weights were determined (right). (c) Tumor volumes were measured at the indicated weeks. (d) Representative images of immunohistochemistry detection of pH3 and PCNA, and TUNEL in mouse tumors of Ptpro−/−/MMTV-Erbb2 and littermate control (left). Bar charts represent the quantification of staining (right). Samples shown are representative of three independent experiments (n=5 per genotype). Error bars indicate s.e.m. *P<0.05, **P<0.01, ***P<0.001 by Student’s t-test. (e) Whole-mount mammary gland preparations (24 W) revealed hyperplasia in Ptpro−/−/MMTV-Erbb2 glands compared with littermate controls. The bottom panels showed a Ptpro−/−/MMTV-Erbb2 gland with pervasive epithelial hyperplasia. (f) Average tumor volume was calculated after initiation of palpable tumors. Error bars indicate s.e.m. (n=6 in Ptpro−/−/MMTV-Erbb2, and 6 in Ptpro+/+/MMTV-Erbb2). *P=0.043, two-sided paired t-test. (g) The box plots of the weights of tumors harvested at 6 weeks are shown. *P=0.047, two-sided paired t-test.
Cell proliferation, as judged by IHC for phospho-S10-histone H3 (phos-H3) and PCNA in sections of dissected tissues, was significantly enhanced in Ptpro−/−/MMTV-Erbb2 vs Ptpro+/+/MMTV-Erbb2 mice (12% vs 6% phos-H3-positive cells, and 23% vs 9% PCNA-positive cells, respectively; P<0.001 for both; Figure 1d). However, there was no statistically significant difference in the level of apoptosis between the two genotypes (Figure 1d). Thus, genetic deletion of Ptpro accelerated the growth rate of Erbb2-driven mammary tumors primarily through stimulating cell proliferation.
In a cross-sectional study of 24-week-old mice of these two genotypes (five mice per group), whole-mount analysis revealed a lower degree of mammary epithelial hyperplasia in Ptpro+/+/MMTV-Erbb2 glands as compared with Ptpro−/−/MMTV-Erbb2 (Figure 1e). We also noticed more mitotic cells in Ptpro−/−/MMTV-Erbb2 tumors as compared with Ptpro+/+/MMTV-Erbb2 tumors in 32-week-old mice (Supplementary Figure 1B). In brief, prevalence of advanced lesions and tumors in Ptpro−/−/MMTV-Erbb2 mice were consistent with the accelerated tumorigenesis due to Ptpro deletion.
To investigate whether the observed accelerated breast tumorigenesis in Ptpro−/−/MMTV-Erbb2 mice was primarily due to loss of Ptpro within the mammary tumor or a consequence of possible off-target effects on systemic circulating factors or non-cancerous host tissues, we transplanted mammary epithelial tissues from both genotypes of Erbb2 transgenic mice when 30 weeks of age. One million primary cells from two genotypic mouse tumors were implanted under the dorsal skin of female nude mice at the right and left flanks, respectively. Ptpro−/−/MMTV-Erbb2 breast cells developed into palpable tumors faster than the Ptpro+/+/MMTV-Erbb2 implanted in the same mouse after implantation (P=0.043, two-sided paired t-test; Figure 1f). The excised Ptpro−/−/MMTV-Erbb2 tumors were significantly heavier than the Ptpro+/+/MMTV-Erbb2 tumors when the mice were killed at 6 weeks after tumor appearance (P=0.047, two-sided paired t-test; Figure 1g). These results indicated that the accelerated mammary tumor development in Ptpro−/−/MMTV-Erbb2 mice was not an off-target consequence due to loss of Ptpro in the whole mouse. Collectively, these in vivo data demonstrated that loss of Ptpro in mammary epithelium caused acceleration of Erbb2-driven breast carcinogenesis and progression.
PTPRO selectively dephosphorylated ERBB2 at Y1248
Although it is known PTPRO can dephosphorylate ERBB2,25 the specific tyrosine residue of ERBB2 that is selectively dephosphorylated by PTPRO is unknown. We thus performed co-immunoprecipitation studies using the SKBR3 cell line transfected with empty vector or PTPRO vector with or without heregulin to stimulate ERBB2 signaling. Precipitated proteins were separated by SDS–polyacrylamide gel electrophoresis followed by immunoblotting with anti-PTPRO, anti-p-ERBB2 (Y877), anti-p-ERBB2 (Y1112), anti-p-ERBB2 (Y1139), anti-p-ERBB2 (Y1196), anti-p-ERBB2 (Y1221) or anti-p-ERBB2 (Y1248) and anti-ERBB2 antibodies (Figure 2a). The ERBB2 associated with immunoprecipitated PTPRO had detectable phosphorylation at several phosphorylation sites but not at Y1248. These data identified that PTPRO dephosphorylated ERBB2 at Y1248.
Interaction of PTPRO with ERBB2 in SKBR3 cells. (a) Co-immunoprecipitation showed interaction between PTPRO and ERBB2. SKBR3 cells were incubated with or without heregulin (HRG). Immunoprecipitation (IP) was performed using either anti-PTPRO or anti-ERBB2 antibodies. Immunoblots of immunoprecipitates were showed with the antigens as labeled to the left. (b) Duolink in situ proximity ligation assay (PLA) in SKBR3 cells. The top two fluorescent micrographs showed the negative controls in which a non-specific immunoglobulins (IgG) replaced either the anti-PTPRO or anti-ERBB2 antibody. Using both anti-PTPRO and anti-ERBB2 antibodies, positive reactions (Red) were detected in cells transfected with scramble control small interfering RNA (siCtrl) while knocking down either ERBB2 or PTPRO with siERBB2 or siPTPRO, respectively, abolished the signals. These results were representative of three independent experiments.
Further, we used proximity ligation assay26 to detect whether there is a direct intermolecular interaction between PTPRO and ERBB2. SKBR3 cells were fixed and incubated with anti-ERBB2 and anti-PTPRO antibodies, followed by Duolink reaction. The results showed that PTPRO and ERBB2 directly interacted at the cell membrane and in the cytoplasm of cancer cells, while knocking down either PTPRO or ERBB2 abolished the interaction (Figure 2b). Together, we for the first time identified the specific tyrosine site of ERBB2 dephosphorylated by PTPRO and the subcellular locations where direct interaction between PPTRO and ERBB2 occurs.
Loss of PTPRO phosphatase activity mimicked PTPRO deletion, leading to increased tumor cell growth
We next examined the cellular mechanisms by which deletion of Ptpro enhanced Erbb2-dependent growth and tumorigenesis. To investigate whether the PTPRO could regulate ERBB2 cellular activities at endogenous expression level, we generated mouse embryonic fibroblasts (MEFs) derived from two genotypic mice and stably transfected them with plasmid FUGW-Erbb2 (Figure 3a). The growth rate was significantly higher in Erbb2/Ptpro−/− MEFs than in Erbb2/Ptpro+/+ MEFs at 48 h (P=0.025) and 72 h (P=0.002; Figure 3b). Further, Erbb2/Ptpro−/− MEFs also showed increased colony formation compared with Erbb2/Ptpro+/+MEFs (P=0.005; Figure 3c). Flow cytometry assay revealed that the percentage of cells in the G1 phase decreased to the much lower level in Erbb2/Ptpro−/− MEFs and this was accompanied by a concomitant increase of accumulated cells in the S phase (Figure 3d), thus indicating blocked cell cycle by PTPRO expression. Meanwhile, Annexin-V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) dual staining assay did not show any difference in apoptosis between the Erbb2/Ptpro+/+ and Erbb2/Ptpro−/− MEFs (Figure 3e).
Effects of Erbb2/Ptpro−/−MEFs on cell viability, cell proliferation and apoptosis. (a) Immunoblotting of two genotypic MEFs derived from littermates, which were stably transfected with FUGW-Erbb2 vector or control vector. (b) Erbb2/Ptpro+/+ MEFs and Erbb2/Ptpro−/− MEFs were inoculated in 96 wells for 24, 48 and 72 h, cell viability was measured by MTT assay. (c) Erbb2/Ptpro+/+ MEFs and Erbb2/Ptpro−/− MEFs cells were seeded in a six-well plate and incubated for 2 weeks to allow colony formation (left). Quantitative determination of colony numbers (right). (d) Cell cycle analysis by flow cytometry in Erbb2/Ptpro+/+ MEFs and Erbb2/Ptpro−/− MEFs. The bar graphs show the relative quantity of G1, G2 and S in the Erbb2/Ptpro+/+ MEFs compared with the Erbb2/Ptpro−/− MEFs. (e) Apoptosis was determined by flow cytometry following Annexin-V-FITC and PI dual labeling. Samples shown are representative of three independent experiments. Error bars indicate s.e.m. *P<0.05, **P<0.01 by Student’s t-test.
To examine the requirement of PTPRO phosphatase activity in the growth of breast cancer cells, we mutated the catalytic site (CS) of PTPRO23 and transfected this plasmid into two ERBB2-positive SKBR3 and BT474 cell lines, which have low PTPRO levels (Figures 4a and b). Transfection of wild-type PTPRO inhibited the proliferation and colony formation of SKBR3 and BT474 cells as compared with empty vector, whereas the CS mutation of PTPRO only partially attenuated its inhibitory activity on cell growth and colony formation (Figures 4c and d). Flow cytometry studies showed a remarkable increase in the percentage of cells in the G1 phase in PTPRO-expressing cells compared with cells transfected with empty vector (control) or the PTPRO-CS mutant (Figure 4e). Flow cytometry for Annexin-V-FITC and PI staining did not show obvious difference in apoptosis among these cells (Figure 4f). These findings suggested that the enhancement of cell proliferation and cell cycle induced by PTPRO deletion was mimicked by the loss of PTPRO phosphatase activity.
Effect of PTPRO overexpression on cell viability, cell proliferation and apoptosis in ERBB2-overexpressing cell lines. (a) Levels of PTPRO protein in 7 breast cancer cell lines examined by immunoblotting. Normal kidney and MCF-10A cells were positive references. (b) Immunoblotting of breast cancer cells transfected with PTPRO-WT vector and mutant PTPRO-CS vector. (c) PTPRO-expressing cells had lower growth rates than that in empty vector and CS mutation of PTPRO cells as indicated by MTT assay. (d) PTPRO-expressing cells had lower clone formation ability than that in empty vector and CS mutation of PTPRO cells. (e) A remarkable increase in percentage of cells in G1 phase in PTPRO-expressing cells compared with cells transfected with empty vector or PTPRO-CS mutant cells as assayed by flow cytometry (left). The bar graphs showed the percentage of cells in G1, G2 and S phase (right). (f) Flow cytometry for Annexin-V-FITC and PI staining did not show visible difference in apoptotic cell distribution among these cells. Samples shown are representative of three independent experiments. Error bars indicate s.e.m. *P<0.05, **P<0.01, ***P<0.001 by Student’s t-test.
ERBB2-dependent oncogenic signaling was antagonized by PTPRO in breast cancer
We next investigated whether PTPRO could regulate ERBB2 phosphorylation and ERBB2-dependent signaling in genetic model, MEFs derived from mice and human cancer cells. Results from the immunoblotting and immunohistochemistry (IHC) studies revealed an increased ERK1/2 (p44/42) phosphorylation as well as AKT phosphorylation in Ptpro−/− mouse mammary tissues and tumors as compared with wild-type controls, indicating the in vivo effects of PTPRO on AKT/ERK signaling (Figures 5a and d and Supplementary Figures 2A and B). In the same line of evidence, Ptpro deletion enhanced ERBB2 signaling and activation of downstream mitogen-activated proteinkinase and phosphatidylinositol 3-kinase/AKT signaling pathways in cultured MEFs overexpressing ERBB2 as compared with wild-type control (Figures 5b and e). Conversely, PTPRO overexpression in SKBR3 and BT474 breast cancer cells was accompanied by reduced phospho-ERK and phospho-AKT signaling as compared with vector control (Figures 5c and f), whereas expression of PTPRO CS-mutant in SKBR3 cells did not impact the activation status of ERK and AKT (Figures 5c and f). Therefore, PTPRO phosphatase activity was required for its impact on ERBB2-AKT-ERK signaling.
PTPRO modulates ERBB2 and downstream signaling through ERK/AKT pathways in breast cancer. (a) Immunoblots of mammary tissues from mice are shown with the antigens labeled on the left. ERBB2 phosphorylation was increased in mammary glands (4 months of age) and tumors (7 months of age) following Ptpro deletion in Erbb2 transgenic mice compared with littermate controls. (b) ERBB2 phosphorylation was increased in Erbb2/Ptpro−/− MEFs compared with littermate controls. (c) ERBB2 phosphorylation was decreased in PTPRO-overexpressing breast cancer cells rather than in PTPRO mutant (CS) cells. (d) Immunoblots of mammary tissues from mice are shown with the antigens labeled on the left. ERK and AKT phosphorylation were increased in mammary glands (4 months of age) and tumors (7 months of age) following Ptpro deletion in Erbb2 transgenic mice compared with littermate controls. (e) ERK and AKT phosphorylation were increased in Erbb2/Ptpro−/− MEFs compared with Erbb2/Ptpro+/+ MEFs. (f) ERK and AKT phosphorylation were decreased in PTPRO-overexpressing breast cancer cells rather than in PTPRO mutant (CS) cells.
In accordance with above findings, correlation analysis of clinical specimen of TMA demonstrated an inverse relationship between PTPRO and the phospho-ERBB2/ERBB2 ratio, phospho-ERK and phospho-AKT levels (PTPRO and phospho-ERBB2/ERBB2 ratio: P<0.001; PTPRO and phospho-ERK: P<0.001; PTPRO and phospho-AKT: P<0.001; Figures 6a and b). Gene set enrichment analysis of published human breast cancer expression profiles27 revealed that PTPRO expression was associated with reduced AKT and ERK signaling gene signatures in ERBB2-positive patients (Figure 6c). Together, these results suggested that PTPRO was an important modulator for ERBB2–AKT–ERK axis both in experimental and clinically relevant settings.
PTPRO expression correlated inversely with p-ERBB2, p-ERK and p-AKT in human breast cancer tissues. (a) Representative immunohistochemistry images for PTPRO, ERBB2, p-ERBB2, p-ERK and p-AKT in five serial sections of the same tumor from two primary human breast cancer specimens (magnification, the top panels: × 100; the bottom panels: × 400). (b) Percentage of samples showing low or high p-ERBB2/ERBB2 ratio, p-ERK and p-AKT expression (from top to bottom) relative to the levels of PTPRO in 180 cases of human breast cancer samples. (c) Gene set enrichment analysis showing inverse correlations between PTPRO expression and an AKT gene signature (CREIGHTON_AKT1_SIGNALING_VIA_MTOR_UP) and an ERK gene signature (BIOCARTA_ERK_PATHWAY) in a published cohort of breast cancer patients (GSE1456; the top tertile (48 cases) of ERBB2 mRNA expression was assumed to be ERBB2-positive tumors). ***P<0.001, Spearman's rank test. FDR, false-discovery rate q value; Neg., negative; NES, normalized enrichment score; Pos., positive.
Since Y1248 is an autophosphorylation site,28 Y1248-phosphorylated ERBB2 might increase as total ERBB2 increases. If the increase in phospho-Y1248 was merely due to increase in total ERBB2, then the ratio of phospho-ERBB2 to total ERBB2 should remain similar. We generated two stable PTPRO-knockdown cells in PTPRO-high ZR75-1 cell line, named ZR75-1-shPTPRO #1 and ZR75-1-shPTPRO #2. Immunoblotting showed that both ZR75-1-shPTPRO #1 and ZR75-1-shPTPRO #2 cells had increased phosphorylation of ERBB2 at Y1248 as compared with ZR75-1-shCtrl both in the basal state and with heregulin stimulation. Importantly, the ratio of phospho-ERBB2 to total ERBB2 noticeably changed upon knockdown of PTPRO, especially when stimulated with heregulin (Supplementary Figure 3). These experiments suggested that loss of PTPRO-mediated dephosphorylation was the main cause of increase in phosphorylation at Y1248.
PTPRO phosphatase activity was required for the endosomal internalization of ERBB2
Given that phosphatases can regulate RTKs at multiple levels through complex mechanisms,29, 30 we thus next investigated whether PTPRO phosphatase activity could control ERBB2 subcellular compartmentalization and internalization and thereby repressed ERBB2 oncogenic function. Flow cytometry assay showed that cell surface ERBB2 was decreased in PTPRO-overexpressing cells, whereas the amount of the cell surface ERBB2 was not obviously altered in CS mutation cells as compared with empty vector-transfected cells (Figure 7a). These results suggested that the internalization and trafficking of ERBB2 might be controlled by PTPRO phosphatase activity to repress ERBB2 signaling.
PTPRO reduced ERBB2 half-life. (a) Cell surface ERBB2 expression was reduced in PTPRO-expressing cells than that in empty vector and CS mutation of PTPRO cells as indicated by flow cytometry. Normal rabbit IgG as a control. Samples shown are representative of three independent experiments. Error bars indicate s.e.m. ***P<0.001 by Student’s t-test. (b) PTPRO-expressing cells were incubated with 20 μg/ml cycloheximide (CHX) for 0, 2, 4, 8, 12 or 24 h plus 10 μmol/l of MG132 or 100 μmol/l of chloroquine. ERBB2 and β-tubulin were detected by immunoblotting and measured by integrated optical density. Half-life (h): Vector: 19.6; PTPRO: 10.2; PTPRO+MG132: 11.8; PTPRO+Chloroquine: 18.0. The log relative optical density (OD) of a band is defined as the log10 (integrated OD of ERBB2/integrated OD of ERBB2 at time 0 h) - log10 (integrated OD of β-tubulin/integrated OD of β-tubulin at time 0 h). (c) PTPRO enhanced endocytosis of ERBB2. Representative confocal fluorescent micrographs are shown. The detected antigens were labeled at the top: ERBB2 and EEA1 (an early endosomes). Areas of co-localization were highlighted using Image J. (d) PTPRO-induced co-localization of ERBB2 and lysosomes. The experiment was similar to (c) except that LAMP-1 (a lysosomal maker) was examined instead of EEA1. DAPI, 4′-6-diamidino-2-phenylindole.
Internalization and degradation are known mechanisms for many RTKs’ downregulation.31 However, little is known for the role of dephosphorylation on the internalization of ERBB2.9, 11, 16, 17 To determine whether the observed PTPRO-mediated downregulation of ERBB2 might result from accelerated protein degradation, SKBR3 cells were treated for 0–24 h with the translation inhibitor cycloheximide to block de novo protein synthesis. PTPRO overexpression remarkably accelerated degradation of ERBB2 (Figure 7b). The half-life of ERBB2 protein decreased to 10.2 h as opposed to 19.6 h in control cells. However, this ERBB2 degradation appeared not to involve proteolysis through the ubiquitin-proteasome system as a proteasome inhibitor MG132 did not attenuate the PTPRO-induced degradation of ERBB2 (Figure 7b). In contrast, a lysosome inhibitor chloroquine abolished ERBB2 degradation in the presence of PTPRO overexpression (Figure 7b), suggesting that the acceleration of ERBB2 degradation by PTPRO was primarily through lysosomal degradation. In corroboration with this evidence, confocal immunofluorescence microscopy demonstrated that the accumulation of ERBB2 increased in endosomes (as shown by co-localization with EEA1) in PTPRO-overexpressing SKBR3 cells (Figure 7c, left panels), whereas CS mutation did not increase such accumulation compared with empty vector control (Figure 7c, left panels). Further, increased ERBB2 was detectable in lysosomes (as shown by LAMP-1 co-localization) of PTPRO-overexpressing cells compared with empty vector or CS mutant cells (Figure 7c, right panels). These data suggested that PTPRO phosphatase activity controlled the endocytotic degradation, contributing to ERBB2 downregulation.
To further address whether phosphorylation of Y1248 was determinant for the lysosomal degradation of ERBB2, we used site-directed mutagenesis to generate dephosphorylated-Y1248-mimicking (that is, mutated to phenylalanine (Y1248F)) and phosphorylated-Y1248-mimicking (that is, mutated to glutamate (Y1248E)) mutants.32 In an ERBB2-negative cell line with low endogenous PTPRO expression (MDA-MB-231), we co-transfected PTPRO-WT or PTPRO-CS with ERBB2-WT or mutants of Y1248, and examined the cellular distribution of ERBB2 Y1248 mutants (Supplementary Figure 4A). As analyzed with confocal immunofluorescence microscopy, dephosphorylation-mimicking mutant of ERBB2, Y1248F, showed co-localization with an endosomal marker (EEA1) and a lysosomal marker (LAMP-1), which was comparable to the results for cells transfected with ERBB2-WT and PTPRO-WT plasmids (Supplementary Figures 4B and C, left panels). Although the loss of phosphatase activity in PTPRO-CS mutant blocked the co-localization with endosomal and lysosomal markers, this had no effect on the dephosphorylation-mimicking mutant (Supplementary Figures 4B and C, right panels). In contrast, the phosphotyrosine-mimicking mutant (Y1248E) did not co-localize with either endosomes or lysosomes in the presence of ectopic expression of PTPRO-WT or PTPRO-CS (Supplementary Figures 4B and C). Together, these data demonstrated that the phosphorylation status of Y1248-regulated endosomal internalization and lysosomal degradation of ERBB2, and dephosphorylation of Y1248 was the mechanism by which PTPRO phosphatase activity regulated these changes in ERBB2.
Therapeutic potentials of epigenetic reexpression of PTPRO for suppressing ERBB2-positive breast cancer
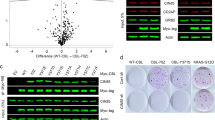
We and others have previously documented PTPRO hypermethylation in breast cancer.24, 33 However, the PTP-associated gene inactivation involved in tumorigenesis and tumor progression may be due to point mutation or deletion, in addition to promoter hypermethylation.34, 35 To test this, we examined the frequency of genomic mutation or deletion in the coding region of PTPRO.36 We analyzed data from the cBioPortal for Cancer Genomics (http://cbioportal.org).37 The incidence of genomic PTPRO mutation was 0.4% of 962 cases (TCGA), 0.2% of 482 cases (TCGA pub) and 1% of 100 cases (Sanger); the rates of PTPRO deletion were 0.6% of 962 cases (TCGA), 0.0% of 482 cases (TCGA pub) and 0.0% of 100 cases (Sanger; Supplementary Figure 5A), whereas PTPRO hypermethylation inversely associated with PTPRO mRNA expression (Supplementary Figure 5B). Given that exon 1 and promoter regions of PTPRO contain typical CpG islands (Figure 8a), we performed methylation-specific PCR using genomic DNA extracted from 37 breast cancer specimens and their respective adjacent non-tumor tissues. In all, 25 (67.6%) of 37 cancer specimens exhibited a complete or partial DNA methylation, whereas 12 (32.4%) of the 37 adjacent non-tumor tissues had partial methylation and no methylation in the remaining non-tumor samples (Supplementary Figure 6A). Furthermore, the PTPRO promoter was hypermethylated in seven breast cancer cell lines, but not in immortalized non-cancerous MCF-10A and normal human mammary tissues (Supplementary Figure 6B). We quantified the relative levels of promoter methylation and protein expression by measuring densitometry of the corresponding bands using software ‘Quantity one’. Pearson’s correlation analysis showed that there was an inverse linear relationships between promoter methylation and protein expression of PTPRO (r=-0.965; P=0.002; Supplementary Figure 6C). These results suggested that promoter hypermethylation, instead of genomic mutation and deletion, was the primary mechanism responsible for repressed PTPRO expression.
5’-Aza-2’-deoxycytidine (5-aza-dC) reduced cell growth that was due to low PTPRO expression. (a) The methylation-specific PCR (MSP) region of the PTPRO gene CpG island. (b) Restored expression of PTPRO by treatment of 5-aza-dC. Expression and methylation analysis of PTPRO in BT474 and SKBR3 cell lines incubated with or without 5-aza-dC. GAPDH was used as an internal loading control. M, methylated; U, unmethylated. (c) Immunoblotting revealed that PTPRO was efficiently knocked down by the treatment of siPTPRO #1 or siPTPRO #2. (d) siPTPRO #1 or siPTPRO #2 cells were treated with and without 5 μM 5-aza-dC for 10 days to allow colony formation (left). Quantitative determination of colony numbers (right). (e) Combination of 5-aza-dC and siPTPRO treatment. SKBR3 cells were treated with siPTPRO #1 for 48 h before incubation with 5 μM 5-aza-dC. Cell lysates were immunoblotted with the labeled antibodies on the left. **P<0.01, ***P<0.001 by Student's t-test.
We next investigated the therapeutic potential that reexpression of PTPRO by epigenetic modification could suppress ERBB2-positive breast cancer. The DNA methyltransferase inhibitor 5’-aza-2’-deoxycytidine (5-aza-dC) was used to block promoter methylation in BT474 and SKBR3 cells. 5-aza-dC treatment of cells decreased methylation, leading to reexpression of PTPRO (Figure 8b). Further, the demethylation treatment using 5-aza-dC effectively reduced the ERBB2-induced cell growth and transformation as evidenced by colony formation (Figures 8c and d), and knockdown of PTPRO expression using small interfering RNA (Figure 8c) diminished the effect of 5-aza-dC (Figure 8d). Thus, the effect of 5-aza-dC was at least in part mediated by reexpression of PTPRO. Moreover, ERBB2-dependent oncogenic signaling was blocked by demethylation treatment using 5-aza-dC (Figure 8e). Taken together, our data strongly argued that reexpression of PTPRO by treatment with 5-aza-dC could suppress ERBB2-positive breast cancer.
Discussion
Although prior epidemiologic, bioinformatics data and cellular studies are suggestive of a tumor-suppressor function for PTPRO in breast cancer,24, 25 definitive in vivo evidence has been lacking until this study. We used a genetic approach by breeding Ptpro-null mice with MMTV-Erbb2 transgenic mice to characterize the role of Ptpro in breast tumorigenesis and cancer progression. Our in vivo results together with in vitro data, firmly establish Ptpro as a tumor-suppressor gene. To exclude the possibility that the noted phenotypes in Ptpro knockout mice could be due to effects of loss of Ptpro in non-breast tissue, we performed transplantation of mammary cells derived from mice. Data derived from tumor cell transplantation strongly argue that the accelerated tumor growth observed in Ptpro−/−/MMTV-Erbb2 mice is directly due to loss of Ptpro function within the breast cancer cells themselves.
It was unclear whether PTPRO repressed ERBB2-positive cancer by blocking the key oncogenic signaling downstream of ERBB2. Using non-malignant immortalized MCF-10A cells, a prior study showed that neither ERK nor AKT was activated by PTPRO downregulation.25 However, we demonstrated that PTPRO regulated AKT and ERK activation in ERBB2-induced cancer cells using genetic mouse model, MEFs derived from transgenic mice, human cancer cells and patient specimens and bioinformatics data mining. The contrasting results from two studies may reflect the difference between non-malignant cells used in prior study and cancer cell systems used in our study. Our studies firmly establish a functional association between PTPRO phosphatase activity and ERBB2 oncogenic signaling using multiple experimental systems. We have verified that PTPRO deficiency promotes ERBB2-driven breast tumorigenesis via activating oncogenic signaling and the phosphatase catalytic site of PTPRO is required for its effect on ERBB2-AKT-ERK signaling. Our studies also highlight a new conceptual framework in which PTPRO downregulates tyrosine phosphorylation of multiple oncogenic pathways in breast carcinogenesis.
Several PTPs have been previously reported to either positively or negatively regulate ERBB2 tyrosine phosphorylation.11, 12, 13, 14, 15, 16, 17 However, structural analysis of the tyrosine phosphatome showed that PTPRO is distinct in structure, catalytic activity and substrate recognition.38 Although ERBB2 has been reported to be the substrate of PTPRO,25 the specific tyrosine residue that is selectively catalyzed by PTPRO remains unknown. In this study, our co-immunoprecipitation experiment along with the results derived from in vivo, cultured cells and clinical specimen confirm that PTPRO dephosphorylates ERBB2 at Y1248. Among the multiple ERBB2 tyrosine phosphorylation sites, Y1248 has been documented to be biologically important and clinically significant. Y1248 has a role in cell differentiation as MUC4/SMC forms a complex with ERBB2, which leads to Y1248 phosphorylation39 and translocation of ERBB2 to the apical surface of polarized epithelial cells.40 In breast cancer, activated Y1248 ERBB2 was detected in 20% of patients,41 and the expression of this phosphorylation site is highly specific for ERBB2 gene amplification.42 Importantly, phosphorylation of Y1248 shows prognostic value; increased ERBB2 phosphorylation of Y1248 represents a lower 5-year disease-free survival rate than patients with low levels phosphorylation of Y1248,43 and Y1248 phosphorylation is an independent predictors of progression-free survival.41 Of note, Y1248 is known to activate mitogen-activated proteinkinase and mediate cell proliferation,42, 44, 45 which supports our findings that PTPRO regulates ERBB2-induced ERK activation and cancer cell proliferation. We also used proximity ligation assay (Duolink) to provide the first evidence that PTPRO and ERBB2 directly bind each other on the cell membrane and in the cytoplasm.
Signaling of RTKs can be regulated at multiple levels. At the receptor level, the quantity of RTKs on the cell surface can be controlled by post-translational modification (for example, dephosphorylation), endocytosis and then degradation via lysosomal or ubiquitin-proteasome system.12, 46 Nevertheless, protein degradation of ERBB2 is poorly understood.16, 47 In this study, we have found that PTPRO regulates the internalization of ERBB2 via endocytosis and subsequent lysosomal degradation. Further, we have revealed that PTPRO catalytic activity is required for the internalization. Thus, PTPRO phosphatase activity is critical for both dephosphorylation and internalization, which is eventually responsible for PTPRO-mediated ERBB2 downregulation (Figure 9).
PTPRO is located on chromosomal region 12p12.3, which is characterized by loss of heterozygosity in different types of cancers.20, 21, 22, 23 In contrast to several other PTPs,34, 48, 49 we have found that PTPRO mutation and deletion are rare in human breast cancer. Given our previous result of high prevalence of PTPRO promoter hypermethylation in breast cancer,24 we conclude that promoter hypermethylation is the major mechanism for PTPRO gene silencing in breast cancer.
Promoter hypermethylation provides a promising molecular target for epigenetic therapy of cancer. As such, we have found that reexpression of PTPRO by treatment with 5-aza-dC can effectively blocked ERBB2-dependent oncogenic signaling as well as tumor growth in cancers with PTPRO silencing. Based on the findings in this study, it is tempting to speculate that reactivation of PTPRO expression through epigenetic modification may be further explored in clinical settings either alone or in combination with targeted therapy. Because loss of PTPRO expression has also been reported in hepatomas,21, 50 colon cancer22, 51 and lung cancer,23 we believe that our molecular insights are likely to be of a broad significance in cancer at-large.
In summary, these studies have established the role of PTPRO as a tumor suppressor in breast cancer in vivo. The model of epigenetic control of PTPRO-mediated ERBB2-AKT-ERK pathway largely reflects the clinical conditions in breast cancer. PTPRO phosphatase activity is required for dephosphorylation, endocytosis and lysosomal degradation of ERBB2, leading to the downregulation of its downstream signaling. The discovered importance of PTPRO in this study will shed light on how to target ERBB2-driven cancers by either selecting the right patients for personalized cancer therapy or designing further therapeutic strategies or both of such approaches.
Materials and methods
Mice
The Ptpro−/−mice, gifts from Dr Bixby, University of Miami, and FVB/N mice carrying the Erbb2 gene (MMTV-Erbb2) were obtained from Jackson Laboratories. All mice were maintained and bred at the Animal Center of Shantou University Medical College. Ptpro−/− mice were bred with wild-type FVB/N mice for 10 generation to obtain Ptpro−/−mice with 99.90% FVB/N background, which took about 2 years, and their female offspring were paired with male MMTV/Erbb2 transgenic mice with a FVB strain background. Age-matched Ptpro+/+/MMTV-Erbb2 and Ptpro−/−/MMTV-Erbb2 virgin mice with 99.95% FVB/N genetic background (11 generation backcross to FVB/N strain) were used for further experiments. Littermates with both genotypes were used in the same phenotypic alterations whenever it was possible. Their genotypes were identified by PCR analyses of tail DNA samples, as described previously.52, 53 The primers of mice genotypes analysis are listed in Supplementary Table 2. Animals were housed in pathogen-free conditions at the Animal Center of Shantou University Medical College in compliance with Institutional Animal Care and Use Committee regulations (SUMC2014-148). All animal experiments were performed according to protocols approved by the Animal Care and Use Committee of the Medical College of Shantou University.
Patient specimens
Surgically treated female breast cancer patients (n=180) with confirmed pathology of invasive ductal carcinoma were collected for preparation of tissue microarray (see Supplementary Information) for IHC and methylation-specific PCR. Breast cancer tissues were obtained from the patients when undergoing surgical treatment at the Department of Surgery, Cancer Hospital of Shantou University Medical College, during the period from 2010 to 2013. All patients received primary treatment by surgery followed by adjuvant radiotherapy, chemotherapy or hormone therapy. A total of 180 primary breast cancer patients contained all subtypes of breast cancer (that is, luminal A, luminal B, ERBB2-enriched and basal-like), including 60 ERBB2-positive patients. Clinical research protocols of this study were reviewed and approved by the Institutional Review Board and the Ethics Committee of Cancer Hospital of Shantou University Medical College (IRB serial number: # 04–070). Written informed consents were obtained from patients in accordance with principles expressed in the Declaration of Helsinki.
References
Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 2009; 27: 1160–1167.
Gradishar WJ . Emerging approaches for treating HER2-positive metastatic breast cancer beyond trastuzumab. Ann Oncol 2013; 24: 2492–2500.
Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L . Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol 2012; 9: 16–32.
Stern HM . Improving treatment of HER2-positive cancers: opportunities and challenges. Sci Transl Med 2012; 4: 127rv122.
Alimandi M, Romano A, Curia MC, Muraro R, Fedi P, Aaronson SA et al. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene 1995; 10: 1813–1821.
Jones RB, Gordus A, Krall JA, MacBeath G . A quantitative protein interaction network for the ErbB receptors using protein microarrays. Nature 2006; 439: 168–174.
Hynes NE, Lane HA . ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 2005; 5: 341–354.
Bard-Chapeau EA, Li S, Ding J, Zhang SS, Zhu HH, Princen F et al. Ptpn11/Shp2 acts as a tumor suppressor in hepatocellular carcinogenesis. Cancer Cell 2011; 19: 629–639.
Sun T, Aceto N, Meerbrey KL, Kessler JD, Zhou C, Migliaccio I et al. Activation of multiple proto-oncogenic tyrosine kinases in breast cancer via loss of the PTPN12 phosphatase. Cell 2011; 144: 703–718.
Aceto N, Sausgruber N, Brinkhaus H, Gaidatzis D, Martiny-Baron G, Mazzarol G et al. Tyrosine phosphatase SHP2 promotes breast cancer progression and maintains tumor-initiating cells via activation of key transcription factors and a positive feedback signaling loop. Nat Med 2012; 18: 529–537.
Gensler M, Buschbeck M, Ullrich A . Negative regulation of HER2 signaling by the PEST-type protein-tyrosine phosphatase BDP1. J Biol Chem 2004; 279: 12110–12116.
Wang HM, Xu YF, Ning SL, Yang DX, Li Y, Du YJ et al. The catalytic region and PEST domain of PTPN18 distinctly regulate the HER2 phosphorylation and ubiquitination barcodes. Cell Res 2014; 24: 1067–1090.
Arias-Romero LE, Saha S, Villamar-Cruz O, Yip SC, Ethier SP, Zhang ZY et al. Activation of Src by protein tyrosine phosphatase 1B Is required for ErbB2 transformation of human breast epithelial cells. Cancer Res 2009; 69: 4582–4588.
Julien SG, Dube N, Read M, Penney J, Paquet M, Han Y et al. Protein tyrosine phosphatase 1B deficiency or inhibition delays ErbB2-induced mammary tumorigenesis and protects from lung metastasis. Nat Genet 2007; 39: 338–346.
Meyer DS, Aceto N, Sausgruber N, Brinkhaus H, Muller U, Pallen CJ et al. Tyrosine phosphatase PTPalpha contributes to HER2-evoked breast tumor initiation and maintenance. Oncogene 2014; 33: 398–402.
Yuan T, Wang Y, Zhao ZJ, Gu H . Protein-tyrosine phosphatase PTPN9 negatively regulates ErbB2 and epidermal growth factor receptor signaling in breast cancer cells. J Biol Chem 2010; 285: 14861–14870.
Zhu JH, Chen R, Yi W, Cantin GT, Fearns C, Yang Y et al. Protein tyrosine phosphatase PTPN13 negatively regulates Her2/ErbB2 malignant signaling. Oncogene 2008; 27: 2525–2531.
Chen B, Bixby JL . A novel substrate of receptor tyrosine phosphatase PTPRO is required for nerve growth factor-induced process outgrowth. J Neurosci 2005; 25: 880–888.
Stepanek L, Sun QL, Wang J, Wang C, Bixby JL . CRYP-2/cPTPRO is a neurite inhibitory repulsive guidance cue for retinal neurons in vitro. J Cell Biol 2001; 154: 867–878.
You YJ, Chen YP, Zheng XX, Meltzer SJ, Zhang H . Aberrant methylation of the PTPRO gene in peripheral blood as a potential biomarker in esophageal squamous cell carcinoma patients. Cancer Lett 2012; 315: 138–144.
Motiwala T, Ghoshal K, Das A, Majumder S, Weichenhan D, Wu YZ et al. Suppression of the protein tyrosine phosphatase receptor type O gene (PTPRO) by methylation in hepatocellular carcinomas. Oncogene 2003; 22: 6319–6331.
Mori Y, Yin J, Sato F, Sterian A, Simms LA, Selaru FM et al. Identification of genes uniquely involved in frequent microsatellite instability colon carcinogenesis by expression profiling combined with epigenetic scanning. Cancer Res 2004; 64: 2434–2438.
Motiwala T, Kutay H, Ghoshal K, Bai S, Seimiya H, Tsuruo T et al. Protein tyrosine phosphatase receptor-type O (PTPRO) exhibits characteristics of a candidate tumor suppressor in human lung cancer. Proc Natl Acad Sci USA 2004; 101: 13844–13849.
Huang YT, Li FF, Ke C, Li Z, Li ZT, Zou XF et al. PTPRO promoter methylation is predictive of poorer outcome for HER2-positive breast cancer: indication for personalized therapy. J Transl Med 2013; 11: 245.
Yu M, Lin G, Arshadi N, Kalatskaya I, Xue B, Haider S et al. Expression profiling during mammary epithelial cell three-dimensional morphogenesis identifies PTPRO as a novel regulator of morphogenesis and ErbB2-mediated transformation. Mol Cell Biol 2012; 32: 3913–3924.
Weibrecht I, Leuchowius KJ, Clausson CM, Conze T, Jarvius M, Howell WM et al. Proximity ligation assays: a recent addition to the proteomics toolbox. Expert Rev Proteomics 2010; 7: 401–409.
Pawitan Y, Bjohle J, Amler L, Borg AL, Egyhazi S, Hall P et al. Gene expression profiling spares early breast cancer patients from adjuvant therapy: derived and validated in two population-based cohorts. Breast Cancer Res 2005; 7: R953–R964.
Chaturvedi P, Singh AP, Chakraborty S, Chauhan SC, Bafna S, Meza JL et al. MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Res 2008; 68: 2065–2070.
Monast CS, Furcht CM, Lazzara MJ . Computational analysis of the regulation of EGFR by protein tyrosine phosphatases. Biophys J 2012; 102: 2012–2021.
Schneeberger VE, Ren Y, Luetteke N, Huang Q, Chen L, Lawrence HR et al. Inhibition of Shp2 suppresses mutant EGFR-induced lung tumors in transgenic mouse model of lung adenocarcinoma. Oncotarget 2015; 6: 6191–6202.
Miaczynska M . Effects of membrane trafficking on signaling by receptor tyrosine kinases. Cold Spring Harb Perspect Biol 2013; 5: a009035.
Anthis NJ, Haling JR, Oxley CL, Memo M, Wegener KL, Lim CJ et al. Beta integrin tyrosine phosphorylation is a conserved mechanism for regulating talin-induced integrin activation. J Biol Chem 2009; 284: 36700–36710.
Ramaswamy B, Majumder S, Roy S, Ghoshal K, Kutay H, Datta J et al. Estrogen-mediated suppression of the gene encoding protein tyrosine phosphatase PTPRO in human breast cancer: mechanism and role in tamoxifen sensitivity. Mol Endocrinol 2009; 23: 176–187.
Zhao S, Sedwick D, Wang Z . Genetic alterations of protein tyrosine phosphatases in human cancers. Oncogene 2015; 34: 3885–3894.
Veeriah S, Brennan C, Meng S, Singh B, Fagin JA, Solit DB et al. The tyrosine phosphatase PTPRD is a tumor suppressor that is frequently inactivated and mutated in glioblastoma and other human cancers. Proc Natl Acad Sci USA 2009; 106: 9435–9440.
Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature 2012; 490: 61–70.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013; 6: pl1.
Barr AJ, Ugochukwu E, Lee WH, King ON, Filippakopoulos P, Alfano I et al. Large-scale structural analysis of the classical human protein tyrosine phosphatome. Cell 2009; 136: 352–363.
Ramsauer VP, Pino V, Farooq A, Carothers Carraway CA, Salas PJ, Carraway KL . Muc4-ErbB2 complex formation and signaling in polarized CACO-2 epithelial cells indicate that Muc4 acts as an unorthodox ligand for ErbB2. Mol Biol Cell 2006; 17: 2931–2941.
Ramsauer VP, Carraway CA, Salas PJ, Carraway KL . Muc4/sialomucin complex, the intramembrane ErbB2 ligand, translocates ErbB2 to the apical surface in polarized epithelial cells. J Biol Chem 2003; 278: 30142–30147.
Hudelist G, Kostler WJ, Czerwenka K, Kubista E, Attems J, Muller R et al. Her-2/neu and EGFR tyrosine kinase activation predict the efficacy of trastuzumab-based therapy in patients with metastatic breast cancer. Int J Cancer 2006; 118: 1126–1134.
Taniyama K, Ishida K, Toda T, Motoshita J, Kuraoka K, Saito A et al. Tyrosine1248-phosphorylated HER2 expression and HER2 gene amplification in female invasive ductal carcinomas. Breast Cancer 2008; 15: 231–240.
Hayashi N, Iwamoto T, Gonzalez-Angulo AM, Ferrer-Lozano J, Lluch A, Niikura N et al. Prognostic impact of phosphorylated HER-2 in HER-2+ primary breast cancer. Oncologist 2011; 16: 956–965.
Dankort D, Jeyabalan N, Jones N, Dumont DJ, Muller WJ . Multiple ErbB-2/Neu phosphorylation sites mediate transformation through distinct effector proteins. J Biol Chem 2001; 276: 38921–38928.
Cicenas J, Urban P, Kung W, Vuaroqueaux V, Labuhn M, Wight E et al. Phosphorylation of tyrosine 1248-ERBB2 measured by chemiluminescence-linked immunoassay is an independent predictor of poor prognosis in primary breast cancer patients. Eur J Cancer 2006; 42: 636–645.
Marmor MD, Yarden Y . Role of protein ubiquitylation in regulating endocytosis of receptor tyrosine kinases. Oncogene 2004; 23: 2057–2070.
Roepstorff K, Grovdal L, Grandal M, Lerdrup M, van Deurs B . Endocytic downregulation of ErbB receptors: mechanisms and relevance in cancer. Histochem Cell Biol 2008; 129: 563–578.
Wang Z, Shen D, Parsons DW, Bardelli A, Sager J, Szabo S et al. Mutational analysis of the tyrosine phosphatome in colorectal cancers. Science 2004; 304: 1164–1166.
Lui VW, Peyser ND, Ng PK, Hritz J, Zeng Y, Lu Y et al. Frequent mutation of receptor protein tyrosine phosphatases provides a mechanism for STAT3 hyperactivation in head and neck cancer. Proc Natl Acad Sci USA 2014; 111: 1114–1119.
Hou J, Xu J, Jiang R, Wang Y, Chen C, Deng L et al. Estrogen-sensitive PTPRO expression represses hepatocellular carcinoma progression by control of STAT3. Hepatology 2013; 57: 678–688.
Asbagh LA, Vazquez I, Vecchione L, Budinska E, De Vriendt V, Baietti MF et al. The tyrosine phosphatase PTPRO sensitizes colon cancer cells to anti-EGFR therapy through activation of SRC-mediated EGFR signaling. Oncotarget 2014; 5: 10070–10083.
Zhang H, Kuang SQ, Liao L, Zhou S, Xu J . Haploid inactivation of the amplified-in-breast cancer 3 coactivator reduces the inhibitory effect of peroxisome proliferator-activated receptor gamma and retinoid X receptor on cell proliferation and accelerates polyoma middle-T antigen-induced mammary tumorigenesis in mice. Cancer Res 2004; 64: 7169–7177.
Zhang H, Singh RR, Talukder AH, Kumar R . Metastatic tumor antigen 3 is a direct corepressor of the Wnt4 pathway. Genes Dev 2006; 20: 2943–2948.
Acknowledgements
This work was supported in part by National Natural Science Foundation of China NSFC (81071736, 30973508 and 81572876 to HZ and 31271495 to HS), the funding for Collaborative and Creative Center, Molecular Diagnosis and Personalized Medicine, Shantou University, Guangdong Province, and the funding from the Department of Education, Guangdong Government under the Top-tier University Development Scheme for Research and Control of Infectious Diseases. We thank Dr Stanley Lin for the careful reading the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Dong, H., Ma, L., Gan, J. et al. PTPRO represses ERBB2-driven breast oncogenesis by dephosphorylation and endosomal internalization of ERBB2. Oncogene 36, 410–422 (2017). https://doi.org/10.1038/onc.2016.213
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.213
This article is cited by
-
Protein tyrosine phosphatase PTPRO represses lung adenocarcinoma progression by inducing mitochondria-dependent apoptosis and restraining tumor metastasis
Cell Death & Disease (2024)
-
Targeting PAK4 reverses cisplatin resistance in NSCLC by modulating ER stress
Cell Death Discovery (2024)
-
Nestin-dependent mitochondria-ER contacts define stem Leydig cell differentiation to attenuate male reproductive ageing
Nature Communications (2022)
-
Monitoring Src status after dasatinib treatment in HER2+ breast cancer with 89Zr-trastuzumab PET imaging
Breast Cancer Research (2018)