Abstract
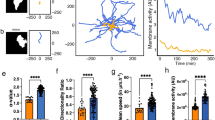
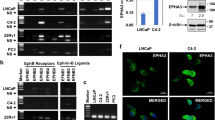
Classical Hodgkin lymphoma (cHL) has a typical clinical manifestation, with dissemination involving functionally neighboring lymph nodes. The factors involved in the spread of lymphoma cells are poorly understood. Here we show that cHL cell lines migrate with higher rates compared with non-Hodgkin lymphoma cell lines. cHL cell migration, invasion and adhesion depend on autocrine WNT signaling as revealed by the inhibition of WNT secretion with the porcupine inhibitors Wnt-C59/IWP-2, but did not affect cell proliferation. While application of recombinant WNT5A or WNT5A overexpression stimulates HL cell migration, neither WNT10A, WNT10B nor WNT16 did so. Time-lapse studies revealed an amoeboid type of cell migration modulated by WNT5A. Reduced migration distances and velocity of cHL cells, as well as altered movement patterns, were observed using porcupine inhibitor or WNT5A antagonist. Knockdown of Frizzled5 and Dishevelled3 disrupted the WNT5A-mediated RHOA activation and cell migration. Overexpression of DVL3-K435M or inhibition of ROCK (Rho-associated protein kinase) by Y-27632/H1152P disrupted cHL cell migration. In addition to these mechanistic insights into the role of WNT5A in vitro, global gene expression data revealed an increased WNT5A expression in primary HL cells in comparison with normal B-cell subsets and other lymphomas. Furthermore, the activity of both porcupine and WNT5A in cHL cells had an impact on lymphoma development in the chick chorionallantoic membrane assay. Massive bleeding of these lymphomas was significantly reduced after inhibition of WNT secretion by Wnt-C59. Therefore, a model is proposed where WNT signaling has an important role in regulating tumor-promoting processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Küppers R, Engert A, Hansmann M-L . Hodgkin lymphoma. J Clin Invest 2012; 122: 3439–3447.
Höpken UE, Foss H-D, Meyer D, Hinz M, Leder K, Stein H et al. Up-regulation of the chemokine receptor CCR7 in classical but not in lymphocyte-predominant Hodgkin disease correlates with distinct dissemination of neoplastic cells in lymphoid organs. Blood 2002; 99: 1109–1116.
Kluk MJ, Ryan KP, Wang B, Zhang G, Rodig SJ, Sanchez T . Sphingosine-1-phosphate receptor 1 in classical Hodgkin lymphoma: assessment of expression and role in cell migration. Lab Invest 2013; 93: 462–471.
Fhu CW, Graham AM, Yap CT, Al-Salam S, Castella A, Chong SM et al. Reed–Sternberg cell-derived lymphotoxin-α activates endothelial cells to enhance T cell recruitment in classical Hodgkin lymphoma. Blood 2014; 124: 2973–2982.
Bargou RC, Emmerich F, Krappmann D, Bommert K, Mapara MY, Arnold W et al. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin’s disease tumor cells. J Clin Invest 1997; 100: 2961–2969.
Jundt F, Anagnostopoulos I, Förster R, Mathas S, Stein H, Dörken B . Activated Notch1 signaling promotes tumor cell proliferation and survival in Hodgkin and anaplastic large cell lymphoma. Blood 2002; 99: 3398–3403.
Zheng B, Fiumara P, Li Y V, Georgakis G, Snell V, Younes M et al. MEK/ERK pathway is aberrantly active in Hodgkin disease : a signaling pathway shared by CD30, CD40, and RANK that regulates cell proliferation and survival. Blood 2003; 102: 1019–1027.
Kube D, Holtick U, Vockerodt M, Ahmadi T, Haier B, Behrmann I et al. STAT3 is constitutively activated in Hodgkin cell lines. Blood 2001; 98: 762–771.
Skinnider BF, Mak TW . The role of cytokines in classical Hodgkin lymphoma. Blood 2002; 99: 4283–4297.
Janz M, Hummel M, Truss M, Wollert-wulf B, Mathas S, Jo K et al. Classical Hodgkin lymphoma is characterized by high constitutive expression of activating transcription factor 3 (ATF3), which promotes viability of Hodgkin/Reed–Sternberg cells. Blood 2006; 107: 2536–2539.
Chiu A, Xu W, He B, Dillon SR, Gross J A, Sievers E et al. Hodgkin lymphoma cells express TACI and BCMA receptors and generate survival and proliferation signals in response to BAFF and APRIL. Blood 2007; 109: 729–739.
Aldinucci D, Lorenzon D, Cattaruzza L, Pinto A, Gloghini A, Carbone A et al. Expression of CCR5 receptors on Reed–Sternberg cells and Hodgkin lymphoma cell lines: involvement of CCL5/Rantes in tumor cell growth and microenvironmental interactions. Int J Cancer 2008; 122: 769–776.
van den Berg A, Visser L, Poppema S . High expression of the CC chemokine TARC in Reed–Sternberg Cells. Am J Pathol 1999; 154: 1685–1691.
Pals ST, Horst E, Scheper RIKJ, Meijer CJLM . Mechanisms of human lymphocyte migration and their role in the pathogenesis of disease. Immunol Rev 1989; 108: 111–133.
Pals ST, de Gorter DJJ, Spaargaren M . Lymphoma dissemination: the other face of lymphocyte homing. Blood 2007; 110: 3102–3111.
Mayor R, Theveneau E . The role of the non-canonical Wnt-planar cell polarity pathway in neural crest migration. Biochem J 2014; 457: 19–26.
Niehrs C . The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol 2012; 13: 767–779.
Lopez-Giral S, Quintana NE, Cabrerizo M, Alfonso-perez M, Sala-Valdes M, Gomez Garcia de Soria V et al. Chemokine receptors that mediate B cell homing to secondary lymphoid tissues are highly expressed in B cell chronic lymphocytic leukemia and non-Hodgkin lymphomas with widespread nodular dissemination. J Leukoc Biol 2004; 76: 462–471.
Xargay-Torrent S, López-Guerra M, Montraveta A, Saborit-Villarroya I, Rosich L, Navarro A et al. Sorafenib inhibits cell migration and stroma-mediated bortezomib resistance by interfering B-cell receptor signaling and protein translation in mantle cell lymphoma. Clin Cancer Res 2013; 19: 586–597.
Kaucká M, Plevová K, Pavlová S, Janovská P, Mishra A, Verner J et al. The planar cell polarity pathway drives pathogenesis of chronic lymphocytic leukemia by the regulation of B-lymphocyte migration. Cancer Res 2013; 73: 1491–1501.
Qiang Y, Walsh K, Yao L, Kedei N, Blumberg PM, Rubin JS et al. Wnts induce migration and invasion of myeloma plasma cells. Blood 2005; 106: 1786–1794.
Wallingford JB, Fraser SE, Harland RM . Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell 2002; 2: 695–706.
Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T et al. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res 2006; 66: 10439–10448.
Qin L, Yin Y, Zheng F, Peng L, Yang C, Bao Y-N et al. WNT5A promotes stemness characteristics in nasopharyngeal carcinoma cells leading to metastasis and tumorigenesis. Oncotarget 2015; 6: 10239–10252.
Klemm F, Bleckmann A, Siam L, Chuang HN, Rietk¨tter E, Behme D et al. β-Catenin-independent WNT signaling in basal-like breast cancer and brain metastasis. Carcinogenesis 2011; 32: 434–442.
Sohlbach K, Moll R, Goßmann J, Nowak O, Barth P, Neubauer A et al. β-Catenin signaling: no relevance in Hodgkin lymphoma? Leuk Lymphoma 2012; 53: 996–998.
Tiacci E, Döring C, Brune V, van Noesel CJM, Klapper W, Mechtersheimer G et al. Analyzing primary Hodgkin and Reed–Sternberg cells to capture the molecular and cellular pathogenesis of classical Hodgkin lymphoma. Blood 2012; 120: 4609–4620.
Brune V, Tiacci E, Pfeil I, Döring C, Eckerle S, van Noesel CJM et al. Origin and pathogenesis of nodular lymphocyte-predominant Hodgkin lymphoma as revealed by global gene expression analysis. J Exp Med 2008; 205: 2251–2268.
Dijksterhuis JP, Baljinnyam B, Stanger K, Sercan HO, Ji Y, Andres O et al. Systematic mapping of WNT-FZD protein interactions reveals functional selectivity by distinct WNT-FZD pairs. J Biol Chem 2015; 290: 6789–6798.
Gonzalez-Sancho JM, Brennan KR, Castelo-soccio LA, Brown AMC . Wnt proteins induce dishevelled phosphorylation via an LRP5/6-independent mechanism, irrespective of their ability to stabilize b-catenin. Mol Cell Biol 2004; 24: 4757–4768.
Bryja V, Schulte G, Rawal N, Grahn A, Arenas E . Wnt-5a induces dishevelled phosphorylation and dopaminergic differentiation via a CK1-dependent mechanism. J Cell Sci 2007; 120: 586–595.
Zhu Y, Tian Y, Du J, Hu Z, Yang L, Liu J et al. Dvl2-dependent activation of Daam1 and RhoA regulates Wnt5a-induced breast cancer cell migration. PLoS One 2012; 7: e37823.
Sahai E, Marshall CJ . Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol 2003; 5: 711–719.
Proffitt KD, Madan B, Ke Z, Pendharkar V, Ding L, Lee MA et al. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res 2013; 73: 502–507.
Long A, Giroux V, Whelan K A, Hamilton KE, Tetreault M-P, Tanaka K et al. WNT10A promotes an invasive and self-renewing phenotype in esophageal squamous cell carcinoma. Carcinogenesis 2015; 36: 598–606.
Aprelikova O, Palla J, Hibler B, Yu X, Greer YE, Yi M et al. Silencing of miR-148a in cancer-associated fibroblasts results in WNT10B-mediated stimulation of tumor cell motility. Oncogene 2013; 32: 3246–3253.
Witze ES, Litman ES, Argast GM, Moon RT, Ahn NG . Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science 2008; 320: 365–369.
Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M et al. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 2002; 1: 279–288.
Jenei V, Sherwood V, Howlin J, Linnskog R, Säfholm A, Axelsson L et al. A t-butyloxycarbonyl-modified Wnt5a-derived hexapeptide functions as a potent antagonist of Wnt5a-dependent melanoma cell invasion. Proc Natl Acad Sci USA 2009; 106: 19473–19478.
Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T et al. Tumor-associated makrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med 2010; 362: 875–885.
Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N . Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. GENES Dev 1998; 12: 2610–2622.
Klingenberg M, Becker J, Eberth S, Kube D, Wilting J . The chick chorioallantoic membrane as an in vivo xenograft model for Burkitt lymphoma. BMC Cancer 2014; 14: 339.
Koch R, Demant M, Aung T, Diering N, Cicholas A, Chapuy B et al. Populational equilibrium through exosome-mediated Wnt signaling in tumor progression of diffuse large B-cell lymphoma. Blood 2014; 123: 2189–2199.
Böll B, Goergen H, Arndt N, Meissner J, Krause SW, Schnell R et al. Relapsed hodgkin lymphoma in older patients: a comprehensive analysis from the German Hodgkin study group. J Clin Oncol 2013; 31: 4431–4437.
Guermazi A, Brice P, de Kerviler EE, Fermé C, Hennequin C, Meignin V et al. Extranodal Hodgkin disease: spectrum of disease. Radiographics 2001; 21: 161–179.
Introcaso CE, Kantor J, Porter DL, Junkins-Hopkins JM . Cutaneous Hodgkin’s disease. J Am Acad Dermatol 2008; 58: 295–298.
Corrigan PM, Dobbin E, Freeburn RW, Wheadon H . Patterns of Wnt/Fzd/LRP gene expression during embryonic hematopoiesis. Stem Cells Dev 2009; 18: 759–772.
Florian MC, Nattamai KJ, Dörr K, Marka G, Uberle B, Vas V et al. A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature 2013; 503: 392–396.
Malhotra S, Baba Y, Garrett KP, Staal FJT, Gerstein R, Kincade PW . Contrasting responses of lymphoid progenitors to canonical and noncanonical Wnt signals. J Immunol 2008; 181: 3955–3964.
Reya T, O’Riordan M, Okamura R, Devaney E, Willert K, Nusse R et al. Wnt signaling regulates B lymphocyte proliferation through a LEF-1 dependent mechanism. Immunity 2000; 13: 15–24.
MacMillan CD, Leong HS, Dales DW, Robertson AE, Lewis JD, Chambers AF et al. Stage of breast cancer progression influences cellular response to activation of the WNT/ planar cell polarity pathway. Sci Rep 2014; 4: 6315.
Gentzel M, Schille C, Rauschenberger V, Schambony A . Distinct functionality of dishevelled isoforms on Ca2+/calmodulin-dependent protein kinase 2 (CamKII) in Xenopus gastrulation. Mol Biol Cell 2015; 26: 966–977.
Kafka A, Tomas D, Beroš V, Pećina HI, Zeljko M, Pećina-Šlaus N . Brain metastases from lung cancer show increased expression of DVL1, DVL3 and beta-catenin and down-regulation of E-cadherin. Int J Mol Sci 2014; 15: 10635–10651.
Samarzija I, Sini P, Schlange T, Macdonald G, Hynes NE . Wnt3a regulates proliferation and migration of HUVEC via canonical and non-canonical Wnt signaling pathways. Biochem Biophys Res Commun 2009; 386: 449–454.
Yao L, Sun B, Zhao XX, Zhao XX, Gu Q, Dong X et al. Overexpression of Wnt5a promotes angiogenesis in NSCLC. Biomed Res Int 2014; 2014: 832562.
Ekström EJ, Bergenfelz C, von Bülow V, Serifler F, Carlemalm E, Jönsson G et al. WNT5A induces release of exosomes containing pro-angiogenic and immunosuppressive factors from malignant melanoma cells. Mol Cancer 2014; 13: 88.
Carragher NO, Walker SM, Scott Carragher LA, Harris F, Sawyer TK, Brunton VG et al. Calpain 2 and Src dependence distinguishes mesenchymal and amoeboid modes of tumour cell invasion: a link to integrin function. Oncogene 2006; 25: 5726–5740.
Vockerodt M, Tesch H, Kube D . Epstein–Barr virus latent membrane protein-1 activates CD25 expression in lymphoma cells involving the NFkappaB pathway. Genes Immun 2001; 2: 433–441.
Kube D, Holtick U, Vockerodt M, Ahmadi T, Haier B, Behrmann I et al. STAT3 is constitutively activated in Hodgkin cell lines. Blood 2001; 98: 762–770.
Najdi R, Proffitt K, Sprowl S, Kaur S, Yu J, Covey TM et al. A uniform human Wnt expression library reveals a shared secretory pathway and unique signaling activities. Differentiation 2012; 84: 203–213.
MacDonald BT, Hien A, Zhang X, Iranloye O, Virshup DM, Waterman ML et al. Disulfide bond requirements for active Wnt ligands. J Biol Chem 2014; 289: 18122–18136.
Bernatík O, Šedová K, Schille C, Ganji RS, Červenka I, Trantírek L et al. Functional analysis of dishevelled-3 phosphorylation identifies distinct mechanisms driven by casein kinase 1ε and frizzled5. J Biol Chem 2014; 289: 23520–23533.
Zepeda-Moreno A, Taubert I, Hellwig I, Hoang V, Pietsch L, Lakshmanan VK et al. Innovative method for quantification of cell-cell adhesion in 96-well plates. Cell Adh Migr 2014; 5: 215–219.
Hecht M, Schulte JH, Eggert A, Wilting J, Schweigerer L . The neurotrophin receptor TrkB cooperates with c-Met in enhancing neuroblastoma invasiveness. Carcinogenesis 2005; 26: 2105–2115.
Acknowledgements
We thank Moritz Harenberg for evaluating the vascularization of the CAM tumors in a blinded manner. We are very thankful to Mrs S Schwoch, Mrs C Zelent and Mrs S Hellbach for their technical assistance in the histological analysis of the CAM tumors. We kindly acknowledge Dr Aldo Ferrari from the ETH Zürich to provide the sketch on the amoeboid migration type (integrated into Figure 3a). This work was supported by grants of the Deutsche Forschungsgemeinschaft Ku 954/12-1 within the Forschergruppe FOR942.
Author contributions
FL, SZ, FvB did most of the experiments with CD, SL, MMN and JW contributing to specific experiments as Micro-CT analysis of the chick chorioallantoic assay, time-lapse experiments, cell track analysis and data interpretation, as well as chick chorioallantoic model characterization. PJ and VB analyzed microarray data from Oncomine. JW, TB, WK, FA, TP and LT were involved in manuscript writing and the final approval. VB designed experiments and wrote the manuscript. FL and DK designed the research, analyzed, interpreted data and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Linke, F., Zaunig, S., Nietert, M. et al. WNT5A: a motility-promoting factor in Hodgkin lymphoma. Oncogene 36, 13–23 (2017). https://doi.org/10.1038/onc.2016.183
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.183
This article is cited by
-
Transcriptome analysis reveals upregulation of immune response pathways at the invasive tumour front of metastatic seminoma germ cell tumours
British Journal of Cancer (2022)
-
FZD5 prevents epithelial-mesenchymal transition in gastric cancer
Cell Communication and Signaling (2021)
-
FZD5 contributes to TNBC proliferation, DNA damage repair and stemness
Cell Death & Disease (2020)
-
Non-canonical WNT-signaling controls differentiation of lymphatics and extension lymphangiogenesis via RAC and JNK signaling
Scientific Reports (2019)
-
Global long terminal repeat activation participates in establishing the unique gene expression programme of classical Hodgkin lymphoma
Leukemia (2019)