Abstract
Mesenchymal stromal cells (MSCs) are a major component of the tumour microenvironment. A plethora of elegant studies focusing on tumour-derived MSCs have shown that they, unlike normal MSCs in other tissue, exhibit a strong ability to promote tumour progression. However, the mechanisms underlying the conversion of normal MSCs into tumour-associated MSCs are unknown. We report here a critical role of tumour cell-derived exosomes in endowing bone marrow-derived MSCs (BM-MSCs) with a tumour-favourable phenotype. Tumour cell-derived exosomes affected neither the growth factor production nor the immunosuppressive property of MSCs; rather, they endowed MSCs with a strong ability to promote macrophage infiltration into B16-F0 melanoma or EL-4 lymphoma. Ablation of macrophages by clodronate liposome administration reversed the tumour-promoting effect of MSCs educated by tumour cell-derived exosomes (TE-MSCs) on the tumour growth. By comparing the chemokine profile of BM-MSCs with that of TE-MSCs, we found that TE-MSCs produced a large amount of CCR2 ligands, CCL2 and CCL7, which are responsible for macrophage recruitment. CCR2-specific inhibitor was found to block the tumour-promoting effect of TE-MSCs. Thus, our investigations demonstrated that tumour cell-derived exosomes confer BM-MSCs the ability to enhance tumour growth. Therefore, we uncovered a novel mechanism underlying the conversion of normal MSCs to tumour-associated MSCs.
Similar content being viewed by others
Introduction
Tumour microenvironment, variably composed of endothelial cells, immune cells, mesenchymal stromal cells (MSCs), fibroblasts, extracellular matrix and soluble factors, has a critical role in orchestrating tumour growth.1, 2 The nonmalignant cellular and acellular components provide a fertile bed for tumour initiation, progression and metastasis.1, 2 Recent studies demonstrated that tumour resident MSCs had an important role in coordinating the tumour microenvironment.3, 4, 5 After mobilized and integrated into the tumour stroma upon sensing the cues of a tumour, MSCs acquire a series of properties to promote tumour growth, including producing growth factors, inhibiting anti-tumour immune responses, shaping tumour inflammatory environment and building the niche for tumour cells.6, 7, 8, 9, 10, 11, 12 Importantly, recent studies revealed that the enhanced tumour promotion ability of tumour-derived MSCs is not intrinsic, rather acquired upon arrival at the tumour microenvironment.3, 4, 7, 11 However, the underlying mechanisms of the conversion of normal MSCs into tumour-favourable MSCs remain unclear.
Exosomes are microvesicles (30–100 nm) originating from the multivesicular bodies and can be isolated from diverse body fluids and cell culture supernatant.13 The contents of exosomes are complex, including various types of proteins, RNAs and lipids and they can act as messengers for cell communication.13, 14, 15 Interestingly, a series of studies have identified exosomes as one of the important mediators of the interaction between tumour cells and their surrounding cells in the tumour microenvironment.16, 17, 18, 19, 20 It has been reported that exosomes from tumour-associated MSCs participate into tumour promotion by transducing micro-RNAs into tumour cells.21, 22 However, the influence of tumour cell-derived exosomes on MSCs is still largely unknown. In the present study, we found that, in comparison with bone marrow-derived MSCs (BM-MSCs), tumour cell-derived exosome-educated MSCs (TE-MSCs) could significantly promote tumour growth. This tumour-promoting effect of TE-MSCs was independent of their growth factor production and the suppression on T cells. Instead, this effect was relied on a vigorous production of CCR2 ligands by TE-MSCs and the resulting recruitment of macrophages into tumour sites. Administration of clodronate liposomes or a CCR2-specific inhibitor could abolish the tumour-promoting effect of TE-MSCs. Therefore, our investigation demonstrated a critical role of tumour cell-derived exosomes in endowing BM-MSCs with tumour promotion capability.
Results and discussion
Various investigations have shown that tumour-associated MSCs can promote tumour growth through released exosomes. However, it is unknown whether tumour cell-derived exosomes could endow BM-MSCs with the capability to promote tumour growth. To this end, we employed the mouse B16-F0 melanoma model. We first isolated exosomes from the culture medium of tumour cells using a commercially available total exosomes isolation kit following the provided protocol. The diameter of the harvested particles was determined by transmission electron microscopy and found to be within range of 30–100 nm (Figure 1a), consistent with the reported diameters of exosomes.13, 15 In addition, these particles were positive for CD9, TSG101 and CD61(Figure 1b), while the intracellular proteins like CYC1, Grp94 and CANX were absent, verifying that the isolated particles were exosomes.13, 15
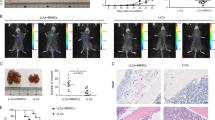
Tumour cell-derived exosomes endowed BM-MSCs with the ability to promote tumour growth in vivo. (a) A representative electron microscope (PHILIPS CM120, Eindhoven, Netherlands) image of tumour cell-derived exosomes. Scale bar, 50 nm. Tumour cell-derived exosomes were isolated from tumour conditioned medium using an exosomes isolation kit (Invitrogen Carlsbad, CA, USA). (b) Tumour cells and tumour cell-derived exosomes were lysed with RIPA lysis buffer (Millipore, Darmstadt, Germany) containing a protease inhibitor cocktail (Sigma, St Louis, MO, USA). Protein concentration was quantified by protein assay (Bio-rad, Hercules, CA, USA), and analysed the exosomes markers CD61 (Cell Signaling, Danvers, MA, USA), CD9 (Cell Signaling), TSG101 (Abcam, Cambridge, MA, USA), CYC1 (Cell Signaling), Grp94 (Cell Signaling) and CANX (Santa Cruz, Dallas, TX, USA). (c) Exosomes derived from tumour cells labelled with PKH26 Dye (Sigma) were cultured with BM-MSCs isolated from GFP transgenic mouse for 24 h. The uptake of exosomes by BM-MSCs was observed under fluorescent microscope. Scale bar, 200 μm. (d) B16-F0 melanoma cells (1 × 106) were inoculated into the hind legs of C57BL/6 mice intramuscularly alone or with BM-MSCs or TE-MSCs (2 × 105), which were derived by culturing BM-MSCs with B16-F0-derived exosomes for 6 days (exosomes were added daily at a final protein concentration of 5 μg/ml). The fetal bovine serum used in our experiments was depleted of exosomes by 18 h ultracentrifugation. Tumours were excised and weighed on days 6, 9 and 12 post tumour inoculation. All procedures were approved by the Institutional Animal Care and Use Committee of the Institute of Health Sciences, Shanghai Institutes for Biological Sciences of Chinese Academy of Sciences. Data was shown as mean±s.d. (n⩾4). Statistical significance was assessed by nonpaired two-tailed Student’s t-test, with significance set as P<0.05; ns, no significance, *P<0.05, ***P<0.001.
Although exosomes have been identified in different experimental systems, the mechanisms that exosomes exert their biological effects are different. Recent studies demonstrated that endocytosis is a critical process for exosomes to deliver messages to target cells.19, 23 To test whether tumour cell-derived exosomes could enter MSCs, we labelled exosomes isolated from tumour cells with red fluorescent lipid dye PKH26 and cultured these labelled exosomes with GFP-MSCs. As shown in Figure 1c, the exosomes were effectively internalized by MSCs.
On the basis of the newly recognized role of exosomes in mediating horizontal transfer of information between cells,20, 21 we hypothesized that tumour cell-derived exosomes are the driving force for the formation of tumour-associated MSCs. To test this hypothesis, we cultured BM-MSCs with or without B16-F0 melanoma cells derived exosomes for 6 days and then inoculated these MSCs together with B16-F0 melanoma cells to the hind limbs of C57BL/6 mice intramuscularly. Strikingly, MSCs cultured with tumour cell-derived exosomes (TE-MSCs) were found to significantly promote the growth of B16-F0 melanoma, while BM-MSCs had little effect (Figure 1d).
Previous studies have revealed that MSCs are rich sources of growth factors, which play an important role in facilitating tumour growth.7, 8, 12 We wondered whether the tumour-promoting effect of TE-MSCs was dependent on the production of growth factors. We thus compared the changes in the expression of growth factors in BM-MSCs and TE-MSCs by employing a growth factor PCR array. Little difference was observed in growth factor expression between TE-MSCs and BM-MSCs (Supplementary Table). In addition, conditioned medium of TE-MSCs exhibited similar effects on B16 melanoma growth as with conditioned medium of BM-MSCs (Supplementary Figure 1a).
Immunoregulation is one of the most prominent properties of MSCs.9, 24 MSC-mediated immunosuppression has been demonstrated to promote tumour growth in many models, such as B16 melanoma and EL-4 lymphoma.25 Therefore, it is possible that TE-MSCs harbour stronger inhibitory effects on T-cell-mediated anti-tumour immune responses. We analysed the types and prevalence of tumour-infiltrated T lymphocytes and found that the percentages of CD4+, CD8+ T cells and Tregs in tumours were not influenced by TE-MSCs (Supplementary Figures 2a-d). We also found in in vitro experimental system that no enhanced suppression on T-cell response was observed in TE-MSCs (Supplementary Figure 1b). Thus, the tumour-promoting effect of TE-MSCs does not rely on their immunosuppressive effect on T cells.
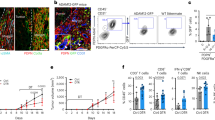
In addition to their potential to regulate T-cell responses, tumour-associated MSCs have been demonstrated to facilitate tumour development through recruiting macrophages and monocytes into tumours.3, 4 We, thus determined the percentage of macrophages in tumour sites and monocytes in peripheral blood from mice with established B16-F0 melanoma by melanoma cell alone, or together with BM-MSCs or TE-MSCs. Flow cytometrical analysis revealed that tumours from mice with TE-MSCs administration exhibited significantly more macrophage (CD11b+F4/80+) accumulation than that from tumours with BM-MSCs inoculation (Figure 2a). As monocytes are considered as precursors of macrophages, we further examined CD11b+Ly6Chigh monocytes in the peripheral blood. Consistently, the higher percentage of CD11b+Ly6Chigh monocytes in the peripheral blood of mice bearing TE-MSCs co-inoculated tumour strongly suggests that these circulating monocytes could contribute to the accumulation of macrophages in the tumour microenvironment (Figure 2b). To verify whether the tumour-promoting effect of TE-MSCs was due to the enhanced macrophage recruitment, we depleted macrophages using clodronate liposomes. We found that following clodronate liposomes administration, the percentage of tumour resident macrophages and circulating monocytes dramatically decreased to the comparable level of mice treated with either TE-MSCs or BM-MSCs alone (Figures 2d and f). Interestingly, depletion of macrophages completely eliminated the promoting effect of TE-MSCs on tumour growth (Figure 2c), suggesting a critical role of macrophages in TE-MSC-mediated tumour promotion. Consistence with previous study,26 clodronate administration was shown to reduce tumour weight in mice injected B16-F0 melanomas alone, further emphasizing the role of macrophages in tumour development.26
The effect of TE-MSCs on tumour growth was dependent on macrophage recruitment. (a and b) B16-F0 melanoma cells (1 × 106) were inoculated into the hind legs of C57BL/6 mice intramuscularly with or without BM-MSCs or TE-MSCs (2 × 105). Nine days post tumour inoculation, single-cell suspensions were prepared from tumour and peripheral blood and analysed for the cell population of CD11b+F4/80+cells and CD11b+ly6Chighcells. All these fluorescence conjugated antibodies were purchased from eBioscience (La Jolla, CA, USA). (c–f) Clodronate liposomes (Encapsula, NanoSciences, Brentwood, TN, USA) and control (Ctrl) liposomes were intravenously injected to B16-F0-bearing mice with BM-MSCs or TE-MSCs injection every other day. Nine days post tumour inoculation, tumours were excised and weighed. Single-cell suspensions prepared from tumour were analysed for the presence of CD11b+F4/80+cells and CD11b±ly6Chighcells by flow cytometry (BD calibur, BD Bioscience, Shanghai, China). Results were representative of three independent experiments. Data was shown as mean±s.d. (more than nine mice in each group). Statistical significance was assessed by nonpaired two-tailed Student’s t-test, with significance set as ns, no significance, *P<0.05, ***P<0.001.
The CCR2 chemotaxis axis is important for macrophage accumulation at tumour sites.4, 27 In comparision with BM-MSCs, we found that TE-MSCs produced higher levels of CCL2 and CCL7 (Figures 3a and b), supporting the observation that more macrophages were recruited to B16-F0 melanoma when injected with TE-MSCs. And the regulation of tumour-derived exosomes on CCL2 and CCL7 expression showed a dose-dependent manner (Supplementary Figure 3). To further test the role of these CCR2 ligands, we employed RS504393, a highly selective CCR2 antagonist, to verify the role of CCR2 ligand-mediated macrophage recruitment in the tumour promotion by TE-MSCs. Interestingly, the tumour-promoting effect and the increased macrophage recruitment by TE-MSCs were both diminished upon RS504393 treatment (Figures 3c and f). Besides, blockade of CCR2 by its inhibitor can reduce the tumour weight in mice with tumour cell injection alone. These results strongly suggest that TE-MSCs recruit more macrophages to tumour sites via producing high level of CCR2 ligands and subsequently facilitate tumour growth.
The dependence of CCR2 ligand production during TE-MSC-mediated recruitment of macrophages. (a) A total of 1 × 103 BM-MSCs or TE-MSCs were seeded in a six-well plate, with the addition of exosomes (at a final concentration of 5 μg/ml) every day. After 6 days incubation, MSCs were collected and analysed for the mRNA expression of CCL2 and CCL7. Data was shown as mean±s.e.m. (b) To analyse the protein levels of CCL2 and CCL7 in BM-MSCs or TE-MSCs, 1 × 105 MSCs were seeded in six-well plate, supernatant was collected after 24 h for ELISA analysis. CCL2 ELISA kit was purchased from R&D (Minneapolis, MN, USA), CCL7 ELISA kit was purchased from eBioscience (La Jolla, CA, USA). Data was shown as mean±s.e.m. (c–f) RS504393 (Sigma), a selective CCR2 inhibitor, was intraperitoneally injected daily (2.5 mg/kg) to B16-F0 melanoma bearing mice with or without BM-MSCs or TE-MSCs coadministration. Tumours were excised and weighed 9 days post tumour inoculation. Single-cell suspensions prepared from tumour were analysed for CD11b+F4/80+ cells and CD11b+ly6Chigh cells by flow cytometry. Results were representative of three independent experiments. Data was shown as mean±s.d. (more than four mice in each group). Statistical significance was assessed by nonpaired two-tailed Student’s t-test, with significance set as ns, no significance, **P<0.01, ***P<0.001.
The role MSCs in tumour progression has been investigated in many studies. MSCs are believed to promote tumour growth through various mechanisms, including growth factor production, immunomodulation and pro-angiogenesis.8, 9, 12 Our study reveals a novel mechanism for the establishment of the tumour-favourable microenvironment by MSCs. In this process, BM-MSCs are enticed by tumour cells released exosomes and evolve into tumour-associated MSCs, with the tumour-promoting capability. This phenomenon can be extended to the EL-4 lymphoma model (Supplementary Figures 4a-c). Therefore, our work provides a new insight into the understanding of the complexity of the interaction between tumour cells and MSCs.
Abbreviations
- BM-MSCs:
-
bone marrow-derived mesenchymal stromal cells
- TE-MSCs:
-
tumour cell-derived exosomes-educated MSCs.
References
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Quail DF, Joyce JA . Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013; 19: 1423–1437.
Ren G, Liu Y, Zhao X, Zhang J, Zheng B, Yuan ZR et al. Tumor resident mesenchymal stromal cells endow naive stromal cells with tumor-promoting properties. Oncogene 2014; 33: 4016–4020.
Ren G, Zhao X, Wang Y, Zhang X, Chen X, Xu C et al. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFalpha. Cell Stem Cell 2012; 11: 812–824.
Cuiffo BG, Campagne A, Bell GW, Lembo A, Orso F, Lien EC et al. MSC-regulated microRNAs converge on the transcription factor FOXP2 and promote breast cancer metastasis. Cell stem cell 2014; 15: 762–774.
Kidd S, Spaeth E, Dembinski JL, Dietrich M, Watson K, Klopp A et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem cells 2009; 27: 2614–2623.
De Boeck A, Pauwels P, Hensen K, Rummens JL, Westbroek W, Hendrix A et al. Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression through paracrine neuregulin 1/HER3 signalling. Gut 2013; 62: 550–560.
Beckermann BM, Kallifatidis G, Groth A, Frommhold D, Apel A, Mattern J et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer 2008; 99: 622–631.
Wang Y, Chen X, Cao W, Shi Y . Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol 2014; 15: 1009–1016.
Lin R, Ma H, Ding Z, Shi W, Qian W, Song J et al. Bone marrow-derived mesenchymal stem cells favor the immunosuppressive T cells skewing in a Helicobacter pylori model of gastric cancer. Stem Cells Dev 2013; 22: 2836–2848.
McLean K, Gong Y, Choi Y, Deng N, Yang K, Bai S et al. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest 2011; 121: 3206–3219.
Huang WH, Chang MC, Tsai KS, Hung MC, Chen HL, Hung SC . Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene 2013; 32: 4343–4354.
Thery C, Zitvogel L, Amigorena S . Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002; 2: 569–579.
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO . Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9: 654–659.
Colombo M, Raposo G, Thery C . Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 2014; 30: 255–289.
Umezu T, Ohyashiki K, Kuroda M, Ohyashiki JH . Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene 2013; 32: 2747–2755.
Webber JP, Spary LK, Sanders AJ, Chowdhury R, Jiang WG, Steadman R et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene 2015; 34: 290–302.
Azmi AS, Bao B, Sarkar FH . Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev 2013; 32: 623–642.
Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 2012; 151: 1542–1556.
Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012; 18: 883–891.
Roccaro AM, Sacco A, Maiso P, Azab AK, Tai YT, Reagan M et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest 2013; 123: 1542–1555.
Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal 2014; 7: ra63.
Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M . Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci USA 2013; 110: 17380–17385.
Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008; 2: 141–150.
Huang Y, Yu P, Li W, Ren G, Roberts AI, Cao W et al. p53 regulates mesenchymal stem cell-mediated tumor suppression in a tumor microenvironment through immune modulation. Oncogene 2014; 33: 3830–3838.
Qian BZ, Pollard JW . Macrophage diversity enhances tumor progression and metastasis. Cell 2010; 141: 39–51.
Arendt LM, McCready J, Keller PJ, Baker DD, Naber SP, Seewaldt V et al. Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Res 2013; 73: 6080–6093.
Acknowledgements
This study was supported by grants from the Scientific Innovation Project of the Chinese Academy of Science (XDA 01040107), the Ministry of Science and Technology of China (2015CB964400), the Programs of National Natural Science of China (81330046, 81273316, 81530043 and 81571612), the External Cooperation Program of BIC, Chinese Academy of Sciences (GJHZ201307), Shanghai Municipal Key Projects of Basic Research (12JC1409200) and Shanghai Rising-Star Program (14QA1404200).
Author contributions
LD and LL designed this project, performed experiments, analysed the data and prepared the manuscript; KC and YH performed experiments and prepared the manuscript; PY, LZ and FL performed experiments; and YW and YS conceived the project, analysed and interpreted the data and drafted the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Lin, L., Du, L., Cao, K. et al. Tumour cell-derived exosomes endow mesenchymal stromal cells with tumour-promotion capabilities. Oncogene 35, 6038–6042 (2016). https://doi.org/10.1038/onc.2016.131
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.131
This article is cited by
-
In vitro study to evaluate the effect of granulocyte colony stimulating factor on colorectal adenocarcinoma and on mesenchymal stem cells trans differentiation into cancer stem cells by cancer cells derived exosomes
Beni-Suef University Journal of Basic and Applied Sciences (2023)
-
Microbiota as the unifying factor behind the hallmarks of cancer
Journal of Cancer Research and Clinical Oncology (2023)
-
Exosomal microRNAs shuttling between tumor cells and macrophages: cellular interactions and novel therapeutic strategies
Cancer Cell International (2022)
-
Lymph node metastasis-derived gastric cancer cells educate bone marrow-derived mesenchymal stem cells via YAP signaling activation by exosomal Wnt5a
Oncogene (2021)
-
Debris-stimulated tumor growth: a Pandora’s box?
Cancer and Metastasis Reviews (2021)