Abstract
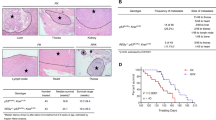
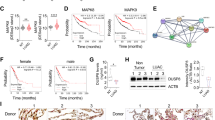
HLJ1 (DNAJB4), a DNAJ/Hsp40 chaperone, has emerged as a novel prognostic marker in lung cancers; however, the molecular contribution and functionality in neoplastic diseases remain to be established. This study demonstrated that HLJ1 inhibits epithelial–mesenchymal transition in vitro and reduces lung cancer metastasis in vivo. Using shRNA silencing and ectopic expression of HLJ1, we found that HLJ1 not only suppresses catalytic activity of Src but also downregulates the formation of oncogenic complexes associated with the EGFR, FAK and STAT3 signaling pathways. A screen of specimens from HLJ1-knockout mice and lung cancer patients validated that HLJ1 expression is inversely correlated with Src activity. Mechanistically, HLJ1 protein directly bound to catalytic and protein-binding domains of Src through its amino acid Y172 and the P301/P304 motif. Following Src-induced HLJ1 phosphorylation at Y172, HLJ1–Src interaction was elevated, resulting in Src inhibition and malignancy suppression. Interestingly, both Src-binding regions also occurred in other DNAJB family members and contributed to anti-invasive activities of DNAJB proteins. We conclude that HLJ1 is an endogenous Src inhibitor that can suppress cancer metastasis through complex interacting mechanisms. This HLJ1–Src complex might provide a promising molecular model for developing new anticancer strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Zhang S, Yu D . Targeting Src family kinases in anti-cancer therapies: turning promise into triumph. Trends Pharmacological Sci 2012; 33: 122–128.
Zhang XH, Wang Q, Gerald W, Hudis CA, Norton L, Smid M et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 2009; 16: 67–78.
Armaiz-Pena GN, Allen JK, Cruz A, Stone RL, Nick AM, Lin YG et al. Src activation by beta-adrenoreceptors is a key switch for tumour metastasis. Nat Commun 2013; 4: 1403.
Zhang J, Kalyankrishna S, Wislez M, Thilaganathan N, Saigal B, Wei W et al. SRC-family kinases are activated in non-small cell lung cancer and promote the survival of epidermal growth factor receptor-dependent cell lines. Am J Pathol 2007; 170: 366–376.
Masaki T, Igarashi K, Tokuda M, Yukimasa S, Han F, Jin YJ et al. pp60c-src activation in lung adenocarcinoma. Eur J Cancer 2003; 39: 1447–1455.
Frame MC . Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta 2002; 1602: 114–130.
Kim LC, Song L, Haura EB . Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol 2009; 6: 587–595.
Rothschild SI, Gautschi O, Haura EB, Johnson FM . Src inhibitors in lung cancer: current status and future directions. Clin Lung Cancer 2010; 11: 238–242.
Johnson FM, Bekele BN, Feng L, Wistuba I, Tang XM, Tran HT et al. Phase II study of dasatinib in patients with advanced non-small-cell lung cancer. J Clin Oncol 2010; 28: 4609–4615.
Brunner AM, Costa DB, Heist RS, Garcia E, Lindeman NI, Sholl LM et al. Treatment-related toxicities in a phase II trial of dasatinib in patients with squamous cell carcinoma of the lung. J Thorac Oncol 2013; 8: 1434–1437.
Chee CE, Krishnamurthi S, Nock CJ, Meropol NJ, Gibbons J, Fu P et al. Phase II study of dasatinib (BMS-354825) in patients with metastatic adenocarcinoma of the pancreas. Oncologist 2013; 18: 1091–1092.
Gonfloni S, Frischknecht F, Way M, Superti-Furga G . Leucine 255 of Src couples intramolecular interactions to inhibition of catalysis. Nat Struct Biol 1999; 6: 760–764.
Johnson LN, Noble ME, Owen DJ . Active and inactive protein kinases: structural basis for regulation. Cell 1996; 85: 149–158.
Erpel T, Superti-Furga G, Courtneidge SA . Mutational analysis of the Src SH3 domain: the same residues of the ligand binding surface are important for intra- and intermolecular interactions. EMBO J 1995; 14: 963–975.
Guarino M . Src signaling in cancer invasion. J Cell Physiol 2010; 223: 14–26.
Saibil H . Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol 2013; 14: 630–642.
Mitra A, Shevde LA, Samant RS . Multi-faceted role of HSP40 in cancer. Clin Exp Metastasis 2009; 26: 559–567.
Sterrenberg JN, Blatch GL, Edkins AL . Human DNAJ in cancer and stem cells. Cancer Lett 2011; 312: 129–142.
Chen CY, Jan CI, Lo JF, Yang SC, Chang YL, Pan SH et al. Tid1-L inhibits EGFR signaling in lung adenocarcinoma by enhancing EGFR Ubiquitinylation and degradation. Cancer Res 2013; 73: 4009–4019.
Ahn BY, Trinh DL, Zajchowski LD, Lee B, Elwi AN, Kim SW . Tid1 is a new regulator of p53 mitochondrial translocation and apoptosis in cancer. Oncogene 2010; 29: 1155–1166.
Mitra A, Menezes ME, Shevde LA, Samant RS . DNAJB6 induces degradation of beta-catenin and causes partial reversal of mesenchymal phenotype. J Biol Chem 2010; 285: 24686–24694.
Mitra A, Menezes ME, Pannell LK, Mulekar MS, Honkanen RE, Shevde LA et al. DNAJB6 chaperones PP2A mediated dephosphorylation of GSK3beta to downregulate beta-catenin transcription target, osteopontin. Oncogene 2012; 31: 4472–4483.
Wang CC, Tsai MF, Hong TM, Chang GC, Chen CY, Yang WM et al. The transcriptional factor YY1 upregulates the novel invasion suppressor HLJ1 expression and inhibits cancer cell invasion. Oncogene 2005; 24: 4081–4093.
Tsai MF, Wang CC, Chang GC, Chen CY, Chen HY, Cheng CL et al. A new tumor suppressor DnaJ-like heat shock protein, HLJ1, and survival of patients with non-small-cell lung carcinoma. J Natl Cancer Inst 2006; 98: 825–838.
Chang TP, Yu SL, Lin SY, Hsiao YJ, Chang GC, Yang PC et al. Tumor suppressor HLJ1 binds and functionally alters nucleophosmin via activating enhancer binding protein 2alpha complex formation. Cancer Res 2010; 70: 1656–1667.
Liu Y, Zhou J, Zhang C, Fu W, Xiao X, Ruan S et al. HLJ1 is a novel biomarker for colorectal carcinoma progression and overall patient survival. Int J Clin Exp Pathol 2014; 7: 969–977.
Chen JJ, Peck K, Hong TM, Yang SC, Sher YP, Shih JY et al. Global analysis of gene expression in invasion by a lung cancer model. Cancer Res 2001; 61: 5223–5230.
Rush J, Moritz A, Lee KA, Guo A, Goss VL, Spek EJ et al. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat Biotechnol 2005; 23: 94–101.
Chen CH, Lin H, Chuang SM, Lin SY, Chen JJ . Acidic stress facilitates tyrosine phosphorylation of HLJ1 to associate with actin cytoskeleton in lung cancer cells. Exp Cell Res 2010; 316: 2910–2921.
Fincham VJ, Unlu M, Brunton VG, Pitts JD, Wyke JA, Frame MC . Translocation of Src kinase to the cell periphery is mediated by the actin cytoskeleton under the control of the Rho family of small G proteins. J Cell Biol 1996; 135: 1551–1564.
Seibel NM, Eljouni J, Nalaskowski MM, Hampe W . Nuclear localization of enhanced green fluorescent protein homomultimers. Anal Biochem 2007; 368: 95–99.
DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014; 64: 252–271.
Boggon TJ, Eck MJ . Structure and regulation of Src family kinases. Oncogene 2004; 23: 7918–7927.
Bibbins KB, Boeuf H, Varmus HE . Binding of the Src SH2 domain to phosphopeptides is determined by residues in both the SH2 domain and the phosphopeptides. Mol Cell Biol 1993; 13: 7278–7287.
Pawson T . Specificity in signal transduction: from phosphotyrosine-SH2 domain interactions to complex cellular systems. Cell 2004; 116: 191–203.
Rahmani Z . APRO4 negatively regulates Src tyrosine kinase activity in PC12 cells. J Cell Sci 2006; 119: 646–658.
Chang BY, Conroy KB, Machleder EM, Cartwright CA . RACK1, a receptor for activated C kinase and a homolog of the beta subunit of G proteins, inhibits activity of src tyrosine kinases and growth of NIH 3T3 cells. Mol Cell Biol 1998; 18: 3245–3256.
Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH et al. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem 1995; 270: 15693–15701.
Oneyama C, Hikita T, Enya K, Dobenecker MW, Saito K, Nada S et al. The lipid raft-anchored adaptor protein Cbp controls the oncogenic potential of c-Src. Mol Cell 2008; 30: 426–436.
Zhou J, Scholes J, Hsieh JT . Characterization of a novel negative regulator (DOC-2/DAB2) of c-Src in normal prostatic epithelium and cancer. J Biol Chem 2003; 278: 6936–6941.
Frame MC . Newest findings on the oldest oncogene; how activated src does it. J Cell Sci 2004; 117: 989–998.
Ishizawar R, Parsons SJ . c-Src and cooperating partners in human cancer. Cancer Cell 2004; 6: 209–214.
Bolos V, Gasent JM, Lopez-Tarruella S, Grande E . The dual kinase complex FAK-Src as a promising therapeutic target in cancer. Onco Targets Ther 2010; 3: 83–97.
Kraskouskaya D, Duodu E, Arpin CC, Gunning PT . Progress towards the development of SH2 domain inhibitors. Chem Soc Rev 2013; 42: 3337–3370.
Chen CH, Statt S, Chiu CL, Thai P, Arif M, Adler KB et al. Targeting myristoylated alanine-rich C kinase substrate phosphorylation site domain in lung cancer. Mechanisms and therapeutic implications. Am J Respir Crit Care Med 2014; 190: 1127–1138.
Zhang S, Huang WC, Li P, Guo H, Poh SB, Brady SW et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med 2011; 17: 461–469.
Acknowledgements
We thank Dr Po-Chao Chan (Department of Life Science, National Chung Hsing University, Taichung, Taiwan) and Dr Samuel Chung (Department of Internal Medicine, University of California Davis, Davis, CA, USA) for useful discussion; Dr Yung-Hao Wong (Institute of Biomedical Sciences, National Chung Hsing University, Taichung, Taiwan) for the advice regarding protein docking; Dr Yu-Ching Lin (UNIMED Healthcare Inc., Taiwan) and the Center for Innovative Therapeutics Discovery of National Taiwan University for technological support in ForteBio system; and the Integrated Core Facility for Functional Genomics of the National Core Facility Program for Biotechnology (NCFPB) for technological support. This work was supported by grants from the Ministry of Science and Technology, Taiwan, ROC (NSC 98-2314-B-005-001-MY3, NSC 102-2911-I-002-303 and NSC 103-2911-I-002-303) and National Taiwan University (102R7557 and 103R7557), as well as in part by the Tobacco-Related Disease Research Program (TRDRP; 23FT-0104 to C-H Chen) and by the Ministry of Education, Taiwan, ROC under the ATU plan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, CH., Chang, WH., Su, KY. et al. HLJ1 is an endogenous Src inhibitor suppressing cancer progression through dual mechanisms. Oncogene 35, 5674–5685 (2016). https://doi.org/10.1038/onc.2016.106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.106
This article is cited by
-
Sodium Butyrate Inhibits the Malignant Proliferation of Colon Cancer Cells via the miR-183/DNAJB4 Axis
Biochemical Genetics (2024)
-
DNAJB4 promotes triple-negative breast cancer cell apoptosis via activation of the Hippo signaling pathway
Discover Oncology (2023)
-
DNAJB4 suppresses breast cancer progression and promotes tumor immunity by regulating the Hippo signaling pathway
Discover Oncology (2023)
-
Identification of a novel signature based on unfolded protein response-related gene for predicting prognosis in bladder cancer
Human Genomics (2021)
-
DNAJB9 suppresses the metastasis of triple-negative breast cancer by promoting FBXO45-mediated degradation of ZEB1
Cell Death & Disease (2021)