Abstract
Dual specificity phosphatase 10 (DUSP10), also known as MAP kinase phosphatase 5 (MKP5), negatively regulates the activation of MAP kinases. Genetic polymorphisms and aberrant expression of this gene are associated with colorectal cancer (CRC) in humans. However, the role of DUSP10 in intestinal epithelial tumorigenesis is not clear. Here, we showed that DUSP10 knockout (KO) mice had increased intestinal epithelial cell (IEC) proliferation and migration and developed less severe colitis than wild-type (WT) mice in response to dextran sodium sulphate (DSS) treatment, which is associated with increased ERK1/2 activation and Krüppel-like factor 5 (KLF5) expression in IEC. In line with increased IEC proliferation, DUSP10 KO mice developed more colon tumours with increased severity compared with WT mice in response to administration of DSS and azoxymethane (AOM). Furthermore, survival analysis of CRC patients demonstrated that high DUSP10 expression in tumours was associated with significant improvement in survival probability. Overexpression of DUSP10 in Caco-2 and RCM-1 cells inhibited cell proliferation. Our study showed that DUSP10 negatively regulates IEC growth and acts as a suppressor for CRC. Therefore, it could be targeted for the development of therapies for colitis and CRC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Podolsky DK . Inflammatory bowel disease. N Engl J Med 2002; 347: 417–429.
Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol 2005; 6: 507–514.
Radtke F, Clevers H . Self-renewal and cancer of the gut: two sides of a coin. Science 2005; 307: 1904–1909.
Koch S, Nava P, Addis C, Kim W, Denning TL, Li L et al. The Wnt antagonist Dkk1 regulates intestinal epithelial homeostasis and wound repair. Gastroenterology 2011; 141: 68 e1–68 e8.
Egan LJ, de Lecea A, Lehrman ED, Myhre GM, Eckmann L, Kagnoff MF et al. Nuclear factor-kappa B activation promotes restitution of wounded intestinal epithelial monolayers. Am J Physiol Cell Physiol 2003; 285: C1028–C1035.
Matsubayashi Y, Ebisuya M, Honjoh S, Nishida E . ERK activation propagates in epithelial cell sheets and regulates their migration during wound healing. Curr Biol 2004; 14: 731–735.
Zhang J, Owen CR, Sanders MA, Turner JR, Basson MD . The motogenic effects of cyclic mechanical strain on intestinal epithelial monolayer wound closure are matrix dependent. Gastroenterology 2006; 131: 1179–1189.
Pai R, Ohta M, Itani RM, Sarfeh IJ, Tarnawski AS . Induction of mitogen-activated protein kinase signal transduction pathway during gastric ulcer healing in rats. Gastroenterology 1998; 114: 706–713.
Lee SH, Hu LL, Gonzalez-Navajas J, Seo GS, Shen C, Brick J et al. ERK activation drives intestinal tumorigenesis in Apc(min/+) mice. Nat Med 2010; 16: 665–670.
Oliveira C, Velho S, Moutinho C, Ferreira A, Preto A, Domingo E et al. KRAS and BRAF oncogenic mutations in MSS colorectal carcinoma progression. Oncogene 2007; 26: 158–163.
Fearon ER . Molecular genetics of colorectal cancer. Annu Rev Pathol 2011; 6: 479–507.
Delaunoit T, Limburg PJ, Goldberg RM, Lymp JF, Loftus EV Jr. . Colorectal cancer prognosis among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2006; 4: 335–342.
Hrabe JE, Byrn JC, Button AM, Zamba GK, Kapadia MR, Mezhir JJ et al. A matched case-control study of IBD-associated colorectal cancer: IBD portends worse outcome. J Surg Oncol 2013; 109: 117–121.
Farooq A, Zhou MM . Structure and regulation of MAPK phosphatases. Cell Signal 2004; 16: 769–779.
Cornell TT, Fleszar A, McHugh W, Blatt NB, Le Vine AM, Shanley TP et al. Mitogen-activated protein kinase phosphatase 2, MKP-2, regulates early inflammation in acute lung injury. Am J Physiol Lung Cell Mol Physiol 2012; 303: L251–L258.
Shi H, Verma M, Zhang L, Dong C, Flavell RA, Bennett AM et al. Improved regenerative myogenesis and muscular dystrophy in mice lacking Mkp5. J Clin Invest 2013. 2064–2077.
Matta R, Barnard JA, Wancket LM, Yan J, Xue J, Grieves J et al. Knockout of Mkp-1 exacerbates colitis in Il-10-deficient mice. Am J Physiol Gastrointest Liver Physiol 2012; 302: G1322–G1335.
Nandan MO, McConnell BB, Ghaleb AM, Bialkowska AB, Sheng H, Shao J et al. Krüppel-like factor 5 mediates cellular transformation during oncogenic KRAS-induced intestinal tumorigenesis. Gastroenterology 2008; 134: 120–130.
Nandan MO, Chanchevalap S, Dalton WB, Yang VW . Krüppel-like factor 5 promotes mitosis by activating the cyclin B1/Cdc2 complex during oncogenic Ras-mediated transformation. FEBS Lett 2005; 579: 4757–4762.
Conkright MD, Wani MA, Anderson KP, Lingrel JB . A gene encoding an intestinal-enriched member of the Krüppel-like factor family expressed in intestinal epithelial cells. Nucleic Acids Res 1999; 27: 1263–1270.
McConnell BB, Kim SS, Bialkowska AB, Yu K, Sitaraman SV, Yang VW et al. Krüppel-like factor 5 protects against dextran sulfate sodium-induced colonic injury in mice by promoting epithelial repair. Gastroenterology 2011; 140: 540–549.e2.
McConnell BB, Kim SS, Yu K, Ghaleb AM, Takeda N, Manabe I et al. Krüppel-like factor 5 is important for maintenance of crypt architecture and barrier function in mouse intestine. Gastroenterology 2011; 141: 1302–1313 1313.e1-6.
Yang Y, Goldstein BG, Nakagawa H, Katz JP . Krüppel-like factor 5 activates MEK/ERK signaling via EGFR in primary squamous epithelial cells. FASEB J 2007; 21: 543–550.
Mori A, Moser C, Lang SA, Hackl C, Gottfried E, Kreutz M et al. Up-regulation of Krüppel-like factor 5 in pancreatic cancer is promoted by interleukin-1beta signaling and hypoxia-inducible factor-1alpha. Mol Cancer Res 2009; 7: 1390–1398.
Usui S, Sugimoto N, Takuwa N, Sakagami S, Takata S, Kaneko S et al. Blood lipid mediator sphingosine 1-phosphate potently stimulates platelet-derived growth factor-A and -B chain expression through S1P1-Gi-Ras-MAPK-dependent induction of Kruppel-like factor 5. J Biol Chem 2004; 279: 12300–12311.
Tanoue T, Moriguchi T, Nishida E . Molecular cloning and characterization of a novel dual specificity phosphatase, MKP-5. J Biol Chem 1999; 274: 19949–19956.
Theodosiou A, Smith A, Gillieron C, Arkinstall S, Ashworth A . MKP5, a new member of the MAP kinase phosphatase family, which selectively dephosphorylates stress-activated kinases. Oncogene 1999; 18: 6981–6988.
Sun R, Chen X, Yang VW . Intestinal-enriched Krüppel-like factor (Krüppel-like factor 5) is a positive regulator of cellular proliferation. J Biol Chem 2001; 276: 6897–6900.
McConnell BB, Bialkowska AB, Nandan MO, Ghaleb AM, Gordon FJ, Yang VW et al. Haploinsufficiency of Krüppel-like factor 5 rescues the tumor-initiating effect of the Apc(Min) mutation in the intestine. Cancer Res 2009; 69: 4125–4133.
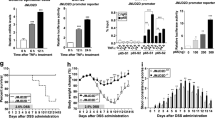
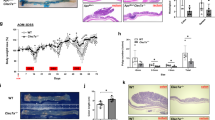
De Robertis M, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L et al. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog 2011; 10: 9.
Bienz M, Clevers H . Linking colorectal cancer to Wnt signaling. Cell 2000; 103: 311–320.
Takahashi M, Nakatsugi S, Sugimura T, Wakabayashi K . Frequent mutations of the beta-catenin gene in mouse colon tumors induced by azoxymethane. Carcinogenesis 2000; 21: 1117–1120.
Takahashi M, Wakabayashi K . Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci 2004; 95: 475–480.
Marisa L, de Reyniès A, Duval A, Selves J, Gaub MP, Vescovo L et al. Gene expression classification of colon cancer into molecular subtypes: characterization, validation, and prognostic value. PLoS Med 2013; 10: e1001453.
Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012; 487: 330–337.
Zhang Y, Blattman JN, Kennedy NJ, Duong J, Nguyen T, Wang Y et al. Regulation of innate and adaptive immune responses by MAP kinase phosphatase 5. Nature 2004; 430: 793–797.
Qian F, Deng J, Gantner BN, Flavell RA, Dong C, Christman JW et al. Map kinase phosphatase 5 protects against sepsis-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 2012; 302: L866–L874.
Kinnebrew MA, Pamer EG . Innate immune signaling in defense against intestinal microbes. Immunol Rev 2012; 245: 113–131.
Sturm A, Dignass AU . Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol 2008; 14: 348–353.
Wang D, Wang H, Brown J, Daikoku T, Ning W, Shi Q et al. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J Exp Med 2006; 203: 941–951.
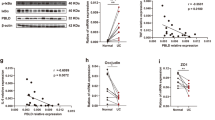
Zhang T, Li X, Du Q, Gong S, Wu M, Mao Z et al. DUSP10 gene polymorphism and risk of colorectal cancer in the Han Chinese population. Eur J Cancer Prev 2014; 23: 173–176.
Houlston RS, Cheadle J, Dobbins SE, Tenesa A, Jones AM, Howarth K et al. Meta-analysis of three genome-wide association studies identifies susceptibility loci for colorectal cancer at 1q41, 3q26.2, 12q13.13 and 20q13.33. Nat Genet 2010; 42: 973–977.
Spain SL, Carvajal-Carmona LG, Howarth KM, Jones AM, Su Z, Cazier JB et al. Refinement of the associations between risk of colorectal cancer and polymorphisms on chromosomes 1q41 and 12q13.13. Hum Mol Genet 2012; 21: 934–946.
Whiffin N, Dobbins SE, Hosking FJ, Palles C, Tenesa A, Wang Y et al. Deciphering the genetic architecture of low-penetrance susceptibility to colorectal cancer. Hum Mol Genet 2013; 22: 5075–5082.
Nomura M, Shiiba K, Katagiri C, Kasugai I, Masuda K, Sato I et al. Novel function of MKP-5/DUSP10, a phosphatase of stress-activated kinases, on ERK-dependent gene expression, and upregulation of its gene expression in colon carcinomas. Oncol Rep 2012; 28: 931–936.
Popovici V, Budinska E, Tejpar S, Weinrich S, Estrella H, Hodgson G et al. Identification of a poor-prognosis BRAF-mutant-like population of patients with colon cancer. J Clin Oncol 2012; 30: 1288–1295.
Gröschl B, Bettstetter M, Giedl C, Woenckhaus M, Edmonston T, Hofstädter F et al. Expression of the MAP kinase phosphatase DUSP4 is associated with microsatellite instability in colorectal cancer (CRC) and causes increased cell proliferation. Int J Cancer 2013; 132: 1537–1546.
Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 2012; 486: 532–536.
Liu Y, Shepherd EG, Nelin LD . MAPK phosphatases—regulating the immune response. Nat Rev Immunol 2007; 7: 202–212.
Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME et al. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med 2006; 203: 131–140.
Cao W, Bao C, Padalko E, Lowenstein CJ . Acetylation of mitogen-activated protein kinase phosphatase-1 inhibits Toll-like receptor signaling. J Exp Med 2008; 205: 1491–1503.
Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med 2008; 5: e54.
Turusov VS, Mohr U International Agency for Research on Cancer, International Life Sciences Institute Pathology of Tumours in Laboratory Animals. 2nd edn International Agency for Research on Cancer: Lyon, 1990.
Foty R . A simple hanging drop cell culture protocol for generation of 3D spheroids. J Vis Exp 2011; 51: 2720–2723.
Zhang X, Wang W, Yu W, Xie Y, Zhang Y, Ma X et al. Development of an in vitro multicellular tumor spheroid model using microencapsulation and its application in anticancer drug screening and testing. Biotechnol Prog 2005; 21: 1289–1296.
Pfaffl MW . A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29: e45.
Schneider CA, Rasband WS, Eliceiri KW . NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9: 671–675.
Chua SW, Vijayakumar P, Nissom PM, Yam CY, Wong VV, Yang H et al. A novel normalization method for effective removal of systematic variation in microarray data. Nucleic Acids Res 2006; 34: e38.
Acknowledgements
We would like to thank Nicholas Gascoigne for advice and helpful discussion. This study was supported by grants from the Office of Deputy President, National University of Singapore, the Ministry of Education (MOE2010-T2-1-079) and the National Medical Research Council (IRG10nov091 and CBRG11nov101) of Singapore.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Png, C., Weerasooriya, M., Guo, J. et al. DUSP10 regulates intestinal epithelial cell growth and colorectal tumorigenesis. Oncogene 35, 206–217 (2016). https://doi.org/10.1038/onc.2015.74
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.74
This article is cited by
-
Identification of specific susceptibility loci for the early-onset colorectal cancer
Genome Medicine (2023)
-
Berberine inhibits intestinal carcinogenesis by suppressing intestinal pro-inflammatory genes and oncogenic factors through modulating gut microbiota
BMC Cancer (2022)
-
PAC1-ing up the epigenetic landscape
Nature Immunology (2020)
-
p38 MAPK activation through B7-H3-mediated DUSP10 repression promotes chemoresistance
Scientific Reports (2019)
-
Colorectal Cancer-Associated Genes Are Associated with Tooth Agenesis and May Have a Role in Tooth Development
Scientific Reports (2018)