Abstract
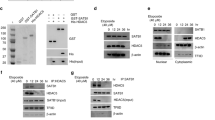
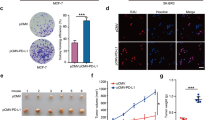
Reduction or loss of tumor-suppressor mammalian STE20-like kinase 1 (MST1) in Hippo pathway contributes to the tumorigenesis. However, the mechanism leading to reduction of MST1 in cancers remains poorly understood. In this study, we explored the hypothesis that the oncoprotein hepatitis B X-interacting protein (HBXIP) is involved in the reduction of MST1 in breast cancer. Immunohistochemical analysis of tissue microarrays revealed that the expression of HBXIP was negatively associated with that of MST1 in 98 clinical breast tissue samples. Then we found that HBXIP could posttranslationally downregulate MST1 in breast cancer cells. Mechanistically, we identified that MST1 could be acetylated on its lysine 35 residue in the cells. Strikingly, the treatment with trichostatin A, an inhibitor of histone deacetylases (HDACs), markedly increased the levels of MST1 acetylation and protein in the cells. Interestingly, the oncoprotein HBXIP could significantly inhibit acetylation of MST1, resulting in the reduction of MST1 protein. Notably, we revealed that the HDAC6 could reduce the protein levels of MST1 through deacetylation modification of MST1 in the cells. Moreover, our data revealed that HBXIP upregulated HDAC6 at the levels of mRNA and protein by activating transcription factor nuclear factor-κB. Deacetylation of MST1 promoted the interaction of MST1 with HSC70 in the cells, resulting in a lysosome-dependent degradation of MST1 via chaperone-mediated autophagy (CMA). Functionally, the reduction of tumor-suppressor MST1 mediated by HBXIP promoted the growth of breast cancer cells in vitro and in vivo. Thus we conclude that the deacetylation of MST1 mediated by HBXIP-enhanced HDAC6 results in MST1 degradation in a CMA manner in promotion of breast cancer growth. Our finding provides new insights into the mechanism of tumor-suppressor MST1 reduction in breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Zhao B, Li L, Lei Q, Guan KL . The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev 2010; 24: 862–874.
Pan D . The Hippo signaling pathway in development and cancer. Dev Cell 2010; 19: 491–505.
Piccolo S, Dupont S, Cordenonsi M . The biology of YAP/TAZ: Hippo signaling and beyond. Physiol Rev 2014; 94: 1287–1312.
Hong W, Guan KL . The YAP and TAZ transcription co-activators: key downstream effectors of the mammalian Hippo pathway. Semin Cell Dev Biol 2012; 23: 785–793.
Li H, Wolfe A, Septer S, Edwards G, Zhong X, Abdulkarim AB et al. Deregulation of Hippo kinase signalling in human hepatic malignancies. Liver Int 2012; 32: 38–47.
Wang P, Mao B, Luo W, Wei B, Jiang W, Liu D et al. The alteration of Hippo/YAP signaling in the development of hypertrophic cardiomyopathy. Basic Res Cardiol 2014; 109: 435.
Steinmann K, Sandner A, Schagdarsurengin U, Dammann RH . Frequent promoter hypermethylation of tumor-related genes in head and neck squamous cell carcinoma. Oncol Rep 2009; 22: 1519–1526.
Minoo P, Zlobec I, Baker K, Tornillo L, Terracciano L, Jass JR et al. Prognostic significance of mammalian sterile 20-like kinase 1 in colorectal cancer. Mod Pathol 2007; 20: 331–338.
Cinar B, Fang PK, Lutchman M, Di Vizio D, Adam RM, Pavlova N et al. The pro-apoptotic kinase Mst1 and its caspase cleavage products are direct inhibitors of Akt1. EMBO J 2007; 26: 4523–4534.
Cinar B, Collak FK, Lopez D, Akgul S, Mukhopadhyay NK, Kilicarslan M et al. MST1 is a multifunctional caspase-independent inhibitor of androgenic signaling. Cancer Res 2011; 71: 4303–4313.
Lin X, Cai F, Li X, Kong X, Xu C, Zuo X et al. Prognostic significance of mammalian sterile 20-like kinase 1 in breast cancer. Tumour Biol 2013; 34: 3239–3243.
Kuser-Abali G, Alptekin A, Cinar B . Overexpression of MYC and EZH2 cooperates to epigenetically silence MST1 expression. Epigenetics 2014; 9: 634–643.
Seidel C, Schagdarsurengin U, Blumke K, Wurl P, Pfeifer GP, Hauptmann S et al. Frequent hypermethylation of MST1 and MST2 in soft tissue sarcoma. Mol Carcinog 2007; 46: 865–871.
Li S, Ran Y, Zhang D, Chen J, Zhu D . MicroRNA-138 plays a role in hypoxic pulmonary vascular remodelling by targeting Mst1. Biochem J 2013; 452: 281–291.
Li K, Wang R, Lozada E, Fan W, Orren DK, Luo J . Acetylation of WRN protein regulates its stability by inhibiting ubiquitination. PLoS One 2010; 5: e10341.
Lv L, Li D, Zhao D, Lin R, Chu Y, Zhang H et al. Acetylation targets the M2 isoform of pyruvate kinase for degradation through chaperone-mediated autophagy and promotes tumor growth. Mol Cell 2011; 42: 719–730.
Zhao D, Zou SW, Liu Y, Zhou X, Mo Y, Wang P et al. Lysine-5 acetylation negatively regulates lactate dehydrogenase A and is decreased in pancreatic cancer. Cancer Cell 2013; 23: 464–476.
de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB . Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 2003; 370: 737–749.
Zhang L, Liu S, Liu N, Zhang Y, Liu M, Li D et al. Proteomic identification and functional characterization of MYH9, Hsc70, and DNAJA1 as novel substrates of HDAC6 deacetylase activity. Protein Cell 2014; 6: 42–54.
Park SJ, Kim JK, Bae HJ, Eun JW, Shen Q, Kim HS et al. HDAC6 sustains growth stimulation by prolonging the activation of EGF receptor through the inhibition of rabaptin-5-mediated early endosome fusion in gastric cancer. Cancer Lett 2014; 354: 97–106.
Saji S, Kawakami M, Hayashi S, Yoshida N, Hirose M, Horiguchi S et al. Significance of HDAC6 regulation via estrogen signaling for cell motility and prognosis in estrogen receptor-positive breast cancer. Oncogene 2005; 24: 4531–4539.
Aldana-Masangkay GI, Sakamoto KM . The role of HDAC6 in cancer. J Biomed Biotechnol 2011; 2011: 875824.
Melegari M, Scaglioni PP, Wands JR . Cloning and characterization of a novel hepatitis B virus X binding protein that inhibits viral replication. J Virol 1998; 72: 1737–1743.
Liu F, You X, Wang Y, Liu Q, Liu Y, Zhang S et al. The oncoprotein HBXIP enhances angiogenesis and growth of breast cancer through modulating FGF8 and VEGF. Carcinogenesis 2014; 35: 1144–1153.
Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM . Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 2012; 150: 1196–1208.
Hu N, Zhang J, Cui W, Kong G, Zhang S, Yue L et al. miR-520b regulates migration of breast cancer cells by targeting hepatitis B X-interacting protein and interleukin-8. J Biol Chem 2011; 286: 13714–13722.
Liu S, Li L, Zhang Y, Zhao Y, You X, Lin Z et al. The oncoprotein HBXIP uses two pathways to up-regulate S100A4 in promotion of growth and migration of breast cancer cells. J Biol Chem 2012; 287: 30228–30239.
Liu Q, Bai X, Li H, Zhang Y, Zhao Y, Zhang X et al. The oncoprotein HBXIP upregulates Lin28B via activating TF II D to promote proliferation of breast cancer cells. Int J Cancer 2013; 133: 1310–1322.
Yue L, Li L, Liu F, Hu N, Zhang W, Bai X et al. The oncoprotein HBXIP activates transcriptional coregulatory protein LMO4 via Sp1 to promote proliferation of breast cancer cells. Carcinogenesis 2013; 34: 927–935.
Zhao B, Ye X, Yu J, Li L, Li W, Li S et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 2008; 22: 1962–1971.
Hsu YL, Hung JY, Chou SH, Huang MS, Tsai MJ, Lin YS et al. Angiomotin decreases lung cancer progression by sequestering oncogenic YAP/TAZ and decreasing Cyr61 expression. Oncogene 2015; 34: 4056–4068.
Salah Z, Melino G, Aqeilan RI . Negative regulation of the Hippo pathway by E3 ubiquitin ligase ITCH is sufficient to promote tumorigenicity. Cancer Res 2011; 71: 2010–2020.
Kang R, Tang D, Schapiro NE, Loux T, Livesey KM, Billiar TR et al. The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor growth by regulating mitochondrial bioenergetics. Oncogene 2014; 33: 567–577.
Ge X, Jin Q, Zhang F, Yan T, Zhai Q . PCAF acetylates {beta}-catenin and improves its stability. Mol Biol Cell 2009; 20: 419–427.
Perez-Mancera PA, Rust AG, van der Weyden L, Kristiansen G, Li A, Sarver AL et al. The deubiquitinase USP9X suppresses pancreatic ductal adenocarcinoma. Nature 2012; 486: 266–270.
Zhang Z, Yamashita H, Toyama T, Sugiura H, Omoto Y, Ando Y et al. HDAC6 expression is correlated with better survival in breast cancer. Clin Cancer Res 2004; 10: 6962–6968.
Ding G, Liu HD, Huang Q, Liang HX, Ding ZH, Liao ZJ et al. HDAC6 promotes hepatocellular carcinoma progression by inhibiting P53 transcriptional activity. FEBS Lett 2013; 587: 880–886.
Madonna R, Geng YJ, Bolli R, Rokosh G, Ferdinandy P, Patterson C et al. Co-activation of nuclear factor-kappaB and myocardin/serum response factor conveys the hypertrophy signal of high insulin levels in cardiac myoblasts. J Biol Chem 2014; 289: 19585–19598.
Montagner M, Enzo E, Forcato M, Zanconato F, Parenti A, Rampazzo E et al. SHARP1 suppresses breast cancer metastasis by promoting degradation of hypoxia-inducible factors. Nature 2012; 487: 380–384.
Mizushima N, Levine B, Cuervo AM, Klionsky DJ . Autophagy fights disease through cellular self-digestion. Nature 2008; 451: 1069–1075.
Jeong H, Then F, Melia Jr TJ, Mazzulli JR, Cui L, Savas JN et al. Acetylation targets mutant huntingtin to autophagosomes for degradation. Cell 2009; 137: 60–72.
Cuervo AM, Knecht E, Terlecky SR, Dice JF . Activation of a selective pathway of lysosomal proteolysis in rat liver by prolonged starvation. Am J Physiol 1995; 269: C1200–C1208.
Majeski AE, Dice JF . Mechanisms of chaperone-mediated autophagy. Int J Biochem Cell Biol 2004; 36: 2435–2444.
Lin CW, Chang YL, Chang YC, Lin JC, Chen CC, Pan SH et al. MicroRNA-135b promotes lung cancer metastasis by regulating multiple targets in the Hippo pathway and LZTS1. Nat Commun 2013; 4: 1877.
Harvey KF, Zhang X, Thomas DM . The Hippo pathway and human cancer. Nat Rev Cancer 2013; 13: 246–257.
Wang FZ, Sha L, Zhang WY, Wu LY, Qiao L, Li N et al. Involvement of hepatitis B X-interacting protein (HBXIP) in proliferation regulation of cells. Acta Pharmacol Sin 2007; 28: 431–438.
Rausa FM 3rd, Hughes DE, Costa RH . Stability of the hepatocyte nuclear factor 6 transcription factor requires acetylation by the CREB-binding protein coactivator. J Biol Chem 2004; 279: 43070–43076.
Jiang W, Wang S, Xiao M, Lin Y, Zhou L, Lei Q et al. Acetylation regulates gluconeogenesis by promoting PEPCK1 degradation via recruiting the UBR5 ubiquitin ligase. Mol Cell 2011; 43: 33–44.
Ropero S, Esteller M . The role of histone deacetylases (HDACs) in human cancer. Mol Oncol 2007; 1: 19–25.
Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012; 150: 780–791.
Ren A, Yan G, You B, Sun J . Down-regulation of mammalian sterile 20-like kinase 1 by heat shock protein 70 mediates cisplatin resistance in prostate cancer cells. Cancer Res 2008; 68: 2266–2274.
Li H, Liu Q, Wang Z, Fang R, Shen Y, Cai X et al. The oncoprotein HBXIP modulates the feedback loop of MDM2/p53 to enhance the growth of breast cancer. J Biol Chem 2015; 290: 22649–22661.
Acknowledgements
This work was supported by grants from the National Basic Research Program of China (973 Program, No. 2015CB553905), the National Natural Scientific Foundation of China (Nos. 81372186, 81272218, 31470756) and Tianjin Natural Scientific Foundation (No. 14JCZDJC32800).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Li, L., Fang, R., Liu, B. et al. Deacetylation of tumor-suppressor MST1 in Hippo pathway induces its degradation through HBXIP-elevated HDAC6 in promotion of breast cancer growth. Oncogene 35, 4048–4057 (2016). https://doi.org/10.1038/onc.2015.476
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.476
This article is cited by
-
Sorafenib triggers ferroptosis via inhibition of HBXIP/SCD axis in hepatocellular carcinoma
Acta Pharmacologica Sinica (2023)
-
Chaperone-mediated autophagy in neurodegenerative diseases: mechanisms and therapy
Molecular and Cellular Biochemistry (2023)
-
The modulation of PD-L1 induced by the oncogenic HBXIP for breast cancer growth
Acta Pharmacologica Sinica (2022)
-
Role of MST1 in the regulation of autophagy and mitophagy: implications for aging-related diseases
Journal of Physiology and Biochemistry (2022)
-
Protein clearance strategies for disease intervention
Journal of Neural Transmission (2022)