Abstract
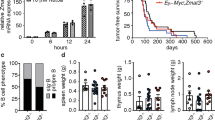
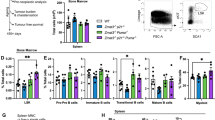
The tumor suppressor p53 is mutated in ~50% of human cancers. P53 is activated by a range of stimuli and regulates several cellular processes, including apoptotic cell death, cell cycle arrest, senescence and DNA repair. P53 induces apoptosis via transcriptional induction of the BH3-only proteins PUMA (p53-upregulated modulator of apoptosis) and NOXA, and cell cycle arrest via p21. Induction of these processes was proposed to be critical for p53-mediated tumor suppression. It is therefore surprising that mice lacking PUMA, NOXA and p21, as well as mice bearing mutations in p53 that impair the transcriptional activation of these genes, are not tumor prone, unlike mice lacking p53 function, which spontaneously develop tumors with 100% incidence. These p53 target genes and the processes they regulate may, however, impact differently on tumor development depending on the oncogenic drivers. For example, loss of PUMA enhances c-MYC-driven lymphoma development in mice, but, interestingly, the acceleration was less impressive compared with that caused by the loss of even a single p53 allele. Different studies have reported that loss of p21 can accelerate, delay or have no impact on tumorigenesis. In an attempt to resolve this controversy, we examined whether loss of p21-mediated cell cycle arrest cooperates with PUMA deficiency in accelerating lymphoma development in Eμ-Myc mice (overexpressing c-MYC in B-lymphoid cells). We found that Eμ-Myc mice lacking both p21 and PUMA (Eμ-Myc;Puma−/−;p21−/−) developed lymphoma at a rate comparable to Eμ-Myc;Puma−/− animals, notably with considerably longer latency than Eμ-Myc;p53+/-mice. Loss of p21 had no impact on the numbers, cycling or survival of pre-leukemic Eμ-Myc B-lymphoid cells, even when PUMA was lost concomitantly. These results demonstrate that even in the context of deregulated c-MYC expression, p53 must suppress tumor development by activating processes apart from, or in addition to, PUMA-mediated apoptosis and p21-induced cell cycle arrest.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lane DP . p53, guardian of the genome. Nature 1992; 358: 15–16.
Levine AJ . p53, the cellular gatekeeper for growth and division. Cell 1997; 88: 323–331.
Vogelstein B, Lane D, Levine AJ . Surfing the p53 network. Nature 2000; 408: 307–310.
Vousden KH, Lane DP . p53 in health and disease. Nat Rev Mol Cell Biol 2007; 8: 275–283.
Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CAJ, Butel JS et al. Mice deficient for p53 are developmentally normal but are susceptible to spontaneous tumours. Nature 1992; 356: 215–221.
Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol 1994; 4: 1–7.
Lowe SW, Sherr CJ . Tumor suppression by Ink4a-Arf: progress and puzzles. Curr Opin Genet Dev 2003; 13: 77–83.
Kamijo T, Weber JD, Zambetti G, Zindy F, Roussel MF, Sherr CJ . Functional and physical interactions of the ARF tumor suppressor with p53 and Mdm2. Proc Natl Acad Sci US A 1998; 95: 8292–8297.
Pomerantz J, Schreiber-Agus N, Liégeois NJ, Silverman A, Alland L, Chin L et al. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell 1998; 92: 713–723.
Zhang Y, Xiong Y, Yarbrough WG . ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell 1998; 92: 725–734.
Michalak EM, Jansen ES, Happo L, Cragg MS, Tai L, Smyth GK et al. Puma and to a lesser extent Noxa are suppressors of Myc-induced lymphomagenesis. Cell Death Differ 2009; 16: 684–696.
Garrison SP, Jeffers JR, Yang C, Nilsson JA, Hall MA, Rehg JE et al. Selection against PUMA gene expression in Myc-driven B-cell lymphomagenesis. Mol Cell Biol 2008; 28: 5391–5402.
Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 1985; 318: 533–538.
Eischen CM, Weber JD, Roussel MF, Sherr CJ, Cleveland JL . Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev 1999; 13: 2658–2669.
Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 2003; 302: 1036–1038.
Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 2003; 4: 321–328.
Hanahan D, Weinberg RA . The hallmarks of cancer. Cell 2000; 100: 57–70.
Deng C, Zhang P, Harper JW, Elledge SJ, Leder P . Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 1995; 82: 675–684.
Newbold A, Salmon JM, Martin BP, Stanley K, Johnstone RW . The role of p21(waf1/cip1) and p27(Kip1) in HDACi-mediated tumor cell death and cell cycle arrest in the Emu-myc model of B-cell lymphoma. Oncogene 2014; 33: 5415–5423.
Patino-Garcia A, Sotillo-Pineiro E, Sierrasesumaga-Ariznabarreta L . p21WAF1 mutation is not a predominant alteration in pediatric bone tumors. Pediatr Res 1998; 43: 393–395.
McKenzie KE, Siva A, Maier S, Runnebaum IB, Seshadri R, Sukumar S . Altered WAF1 genes do not play a role in abnormal cell cycle regulation in breast cancers lacking p53 mutations. Clin Cancer Res 1997; 3: 1669–1673.
Shiohara M, el-Deiry WS, Wada M, Nakamaki T, Takeuchi S, Yang R et al. Absence of WAF1 mutations in a variety of human malignancies. Blood 1994; 84: 3781–3784.
Jackson RJ, Adnane J, Coppola D, Cantor A, Sebti SM, Pledger WJ . Loss of the cell cycle inhibitors p21(Cip1) and p27(Kip1) enhances tumorigenesis in knockout mouse models. Oncogene 2002; 21: 8486–8497.
Topley GI, Okuyama R, Gonzales JG, Conti C, Dotto GP . p21(WAF1/Cip1) functions as a suppressor of malignant skin tumor formation and a determinant of keratinocyte stem-cell potential. Proc Natl Acad Sci USA 1999; 96: 9089–9094.
Adnane J, Jackson RJ, Nicosia SV, Cantor AB, Pledger WJ, Sebti SM . Loss of p21WAF1/CIP1 accelerates Ras oncogenesis in a transgenic/knockout mammary cancer model. Oncogene 2000; 19: 5338–5347.
Yang WC, Mathew J, Velcich A, Edelmann W, Kucherlapati R, Lipkin M et al. Targeted inactivation of the p21(WAF1/cip1) gene enhances Apc-initiated tumor formation and the tumor-promoting activity of a Western-style high-risk diet by altering cell maturation in the intestinal mucosal. Cancer Res 2001; 61: 565–569.
Wang YA, Elson A, Leder P . Loss of p21 increases sensitivity to ionizing radiation and delays the onset of lymphoma in atm-deficient mice. Proc Natl Acad Sci USA 1997; 94: 14590–14595.
Martin-Caballero J, Flores JM, Garcia-Palencia P, Serrano M . Tumor susceptibility of p21(Waf1/Cip1)-deficient mice. Cancer Res 2001; 61: 6234–6238.
Bearss DJ, Lee RJ, Troyer DA, Pestell RG, Windle JJ . Differential effects of p21(WAF1/CIP1) deficiency on MMTV-ras and MMTV-myc mammary tumor properties. Cancer Res 2002; 62: 2077–2084.
Martins CP, Berns A . Loss of p27(Kip1) but not p21(Cip1) decreases survival and synergizes with MYC in murine lymphomagenesis. EMBO J 2002; 21: 3739–3748.
Barboza JA, Liu G, Ju Z, El-Naggar AK, Lozano G . p21 delays tumor onset by preservation of chromosomal stability. Proc Natl Acad Sci USA 2006; 103: 19842–19847.
Post SM, Quintas-Cardama A, Terzian T, Smith C, Eischen CM, Lozano G . p53-dependent senescence delays Emu-myc-induced B-cell lymphomagenesis. Oncogene 2010; 29: 1260–1269.
Valente LJ, Gray DH, Michalak EM, Pinon-Hofbauer J, Egle A, Scott CL et al. p53 efficiently suppresses tumor development in the complete absence of its cell-cycle inhibitory and proapoptotic effectors p21, Puma, and Noxa. Cell Rep 2013; 3: 1339–1345.
Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y et al. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell 2012; 149: 1269–1283.
Aubrey BJ, Kelly GL, Kueh AJ, Brennan MS, O'Connor L, Milla L et al. An inducible lentiviral guide rna platform enables the identification of tumor-essential genes and tumor-promoting mutations in vivo. Cell Rep 2015; 10: 1422–1432.
Harris AW, Pinkert CA, Crawford M, Langdon WY, Brinster RL, Adams JM . The Eμ-myc transgenic mouse: a model for high-incidence spontaneous lymphoma and leukemia of early B cells. J Exp Med 1988; 167: 353–371.
Langdon WY, Harris AW, Cory S, Adams JM . The c-myc oncogene perturbs B lymphocyte development in Eμ-myc transgenic mice. Cell 1986; 47: 11–18.
Strasser A, Elefanty AG, Harris AW, Cory S . Progenitor tumours from Em-bcl-2-myc transgenic mice have lymphomyeloid differentiation potential and reveal developmental differences in cell survival. EMBO J 1996; 15: 3823–3834.
Strasser A, Harris AW, Bath ML, Cory S . Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature 1990; 348: 331–333.
Nakano K, Vousden KH . PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 2001; 7: 683–694.
Jiang D, Brady CA, Johnson TM, Lee EY, Park EJ, Scott MP et al. Full p53 transcriptional activation potential is dispensable for tumor suppression in diverse lineages. Proc Natl Acad Sci USA 2011; 108: 17123–17128.
Hoffman B, Liebermann DA . Apoptotic signaling by c-MYC. Oncogene 2008; 27: 6462–6472.
Bieging KT, Attardi LD . Deconstructing p53 transcriptional networks in tumor suppression. Trends Cell Biol 2012; 22: 97–106.
Riley T, Sontag E, Chen P, Levine A . Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol 2008; 9: 402–412.
Brady CA, Jiang D, Mello SS, Johnson TM, Jarvis LA, Kozak MM et al. Distinct p53 transcriptional programs dictate acute DNA-damage responses and tumor suppression. Cell 2011; 145: 571–583.
Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ . Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 1995; 377: 552–557.
Acknowledgements
The authors thank Drs GL Kelly, ARD Delbridge, BJ Aubrey, S Cory and JM Adams for discussions and gifts of mice; K Walker, J Mansheim, C Gatt, G Siciliano and S O’Connor for expert animal care; B Helbert, H Ierino, K Mackwell and C Young for help with genotyping; J Corbin and J McManus for automated blood cell analysis. This work was supported by a PhD scholarship and Postdoctoral Fellowships from the Cancer Council of Victoria to SG and LV, by Postdoctoral Fellowships from the Leukaemia Foundation Australia to SG, from the Lady Tata Memorial Trust Research Award to AJ and SG, from the Leukemia & Lymphoma Society of America and the Beatriu de Pinos Foundation to AJ and by grants from Cancer Australia and Cure Cancer Australia Foundation (grant no. 1067571), the National Health and Medical Research Council (NHMRC; program grant no. 1016701, NHMRC Senior Principal Research Fellow (SPRF) Fellowship 1020363 to AS) and the Leukemia & Lymphoma Society of America (Specialized Center of Research (SCOR) grant no. 7001-13). This work was made possible by operational infrastructure grants through the Australian Government Independent Medical Research Institutes Infrastructure Support Scheme (IRIISS) and the Victorian State Government Operational Infrastructure Support (OIS).
Author contributions
LV, SG, AJ and AS conceived the study, planned the experiments, interpreted the results and prepared the manuscript. LV, AJ, CV and SG conducted the majority of the experiments.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Valente, L., Grabow, S., Vandenberg, C. et al. Combined loss of PUMA and p21 accelerates c-MYC-driven lymphoma development considerably less than loss of one allele of p53. Oncogene 35, 3866–3871 (2016). https://doi.org/10.1038/onc.2015.457
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.457
This article is cited by
-
Consequences of Zmat3 loss in c-MYC- and mutant KRAS-driven tumorigenesis
Cell Death & Disease (2020)
-
ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway
Nature Cell Biology (2019)
-
How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression?
Cell Death & Differentiation (2018)
-
Cell cycle arrest through indirect transcriptional repression by p53: I have a DREAM
Cell Death & Differentiation (2018)
-
DNA repair processes are critical mediators of p53-dependent tumor suppression
Nature Medicine (2018)