Abstract
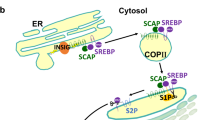
Sterol-regulatory element-binding proteins (SREBPs) are key transcription factors regulating cholesterol and fatty acid biosynthesis. SREBP activity is tightly regulated to maintain lipid homeostasis, and is modulated upon extracellular stimuli such as growth factors. While the homeostatic SREBP regulation is well studied, stimuli-dependent regulatory mechanisms are still elusive. Here we demonstrate that SREBPs are regulated by a previously uncharacterized mechanism through transforming growth factor-β activated kinase 1 (TAK1), a signaling molecule of inflammation. We found that TAK1 binds to and inhibits mature forms of SREBPs. In an in vivo setting, hepatocyte-specific Tak1 deletion upregulates liver lipid deposition and lipogenic enzymes in the mouse model. Furthermore, hepatic Tak1 deficiency causes steatosis pathologies including elevated blood triglyceride and cholesterol levels, which are established risk factors for the development of hepatocellular carcinoma (HCC) and are indeed correlated with Tak1-deficiency-induced HCC development. Pharmacological inhibition of SREBPs alleviated the steatosis and reduced the expression level of the HCC marker gene in the Tak1-deficient liver. Thus, TAK1 regulation of SREBP critically contributes to the maintenance of liver homeostasis to prevent steatosis, which is a potentially important mechanism to prevent HCC development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Horton JD, Goldstein JL, Brown MS . SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 2002; 109: 1125–1131.
Brown MS, Goldstein JL . The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 1997; 89: 331–340.
Shimano H, Shimomura I, Hammer RE, Herz J, Goldstein JL, Brown MS et al. Elevated levels of SREBP-2 and cholesterol synthesis in livers of mice homozygous for a targeted disruption of the SREBP-1 gene. J Clin Invest 1997; 100: 2115–2124.
Yang T, Espenshade PJ, Wright ME, Yabe D, Gong Y, Aebersold R et al. Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell 2002; 110: 489–500.
Goldstein JL, DeBose-Boyd RA, Brown MS . Protein sensors for membrane sterols. Cell 2006; 124: 35–46.
Sundqvist A, Ericsson J . Transcription-dependent degradation controls the stability of the SREBP family of transcription factors. Proc Natl Acad Sci USA 2003; 100: 13833–13838.
Sundqvist A, Bengoechea-Alonso MT, Ye X, Lukiyanchuk V, Jin J, Harper JW et al. Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCF(Fbw7). Cell Metab 2005; 1: 379–391.
Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 2011; 146: 408–420.
Laplante M, Sabatini DM . mTORC1 activates SREBP-1c and uncouples lipogenesis from gluconeogenesis. Proc Natl Acad Sci USA 2010; 107: 3281–3282.
Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell 2010; 39: 171–183.
Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab 2008; 8: 224–236.
Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab 2011; 14: 21–32.
Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell 2000; 6: 1355–1364.
Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest 2009; 119: 1201–1215.
Schuck S, Prinz WA, Thorn KS, Voss C, Walter P . Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. J Cell Biol 2009; 187: 525–536.
Ron D, Hampton RY . Membrane biogenesis and the unfolded protein response. J Cell Biol 2004; 167: 23–25.
Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 2011; 13: 376–388.
Mihaly SR, Ninomiya-Tsuji J, Morioka S . TAK1 control of cell death. Cell Death Differ 2014; 21: 1667–1676.
Morioka S, Inagaki M, Komatsu Y, Mishina Y, Matsumoto K, Ninomiya-Tsuji J . TAK1 kinase signaling regulates embryonic angiogenesis by modulating endothelial cell survival and migration. Blood 2012; 120: 3846–3857.
Omori E, Matsumoto K, Sanjo H, Sato S, Akira S, Smart RC et al. TAK1 is a master regulator of epidermal homeostasis involving skin inflammation and apoptosis. J Biol Chem 2006; 281: 19610–19617.
Kajino-Sakamoto R, Inagaki M, Lippert E, Akira S, Robine S, Matsumoto K et al. Enterocyte-derived TAK1 signaling prevents epithelium apoptosis and the development of ileitis and colitis. J Immunol 2008; 181: 1143–1152.
Ikeda Y, Morioka S, Matsumoto K, Ninomiya-Tsuji J . TAK1 binding protein 2 is essential for liver protection from stressors. PLoS ONE 2014; 9: e88037.
Inokuchi S, Aoyama T, Miura K, Osterreicher CH, Kodama Y, Miyai K et al. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc Natl Acad Sci USA 2010; 107: 844–849.
Bettermann K, Vucur M, Haybaeck J, Koppe C, Janssen J, Heymann F et al. TAK1 suppresses a NEMO-dependent but NF-κB-independent pathway to liver cancer. Cancer Cell 2010; 17: 481–496.
Morioka S, Broglie P, Omori E, Ikeda Y, Takaesu G, Matsumoto K et al. TAK1 kinase switches cell fate from apoptosis to necrosis following TNF stimulation. J Cell Biol 2014; 204: 607–623.
Yang L, Inokuchi S, Roh YS, Song J, Loomba R, Park EJ et al. Transforming growth factor-β signaling in hepatocytes promotes hepatic fibrosis and carcinogenesis in mice with hepatocyte-specific deletion of TAK1. Gastroenterology 2013; 144: 1042–1054 e1044.
Das M, Garlick DS, Greiner DL, Davis RJ . The role of JNK in the development of hepatocellular carcinoma. Genes Dev 2011; 25: 634–645.
Wagner EF, Nebreda AR . Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer 2009; 9: 537–549.
Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R et al. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell 2007; 11: 119–132.
Maeda S, Kamata H, Luo JL, Leffert H, Karin M . IKKβ couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 2005; 121: 977–990.
Karin M . Nuclear factor-κB in cancer development and progression. Nature 2006; 441: 431–436.
Sohda T, Iwata K, Soejima H, Kamimura S, Shijo H, Yun K . In situ detection of insulin-like growth factor II (IGF2) and H19 gene expression in hepatocellular carcinoma. J Hum Genet 1998; 43: 49–53.
Matouk IJ, DeGroot N, Mezan S, Ayesh S, Abu-lail R, Hochberg A et al. The H19 non-coding RNA is essential for human tumor growth. PLoS ONE 2007; 2: e845.
Braeuning A, Jaworski M, Schwarz M, Kohle C . Rex3 (reduced in expression 3) as a new tumor marker in mouse hepatocarcinogenesis. Toxicology 2006; 227: 127–135.
Inokuchi-Shimizu S, Park EJ, Roh YS, Yang L, Zhang B, Song J et al. TAK1-mediated autophagy and fatty acid oxidation prevent hepatosteatosis and tumorigenesis. J Clin Invest 2014; 124: 3566–3578.
Cohen JC, Horton JD, Hobbs HH . Human fatty liver disease: old questions and new insights. Science 2011; 332: 1519–1523.
Browning JD, Horton JD . Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 2004; 114: 147–152.
Sabio G, Cavanagh-Kyros J, Ko HJ, Jung DY, Gray S, Jun JY et al. Prevention of steatosis by hepatic JNK1. Cell Metab 2009; 10: 491–498.
Momcilovic M, Hong SP, Carlson M . Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem 2006; 281: 25336–25343.
Kajino T, Omori E, Ishii S, Matsumoto K, Ninomiya-Tsuji J . TAK1 MAPK kinase kinase mediates transforming growth factor-β signaling by targeting SnoN oncoprotein for degradation. J Biol Chem 2007; 282: 9475–9481.
Takaesu G, Kishida S, Hiyama A, Yamaguchi K, Shibuya H, Irie K et al. TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol Cell 2000; 5: 649–658.
Kishimoto K, Matsumoto K, Ninomiya-Tsuji J . TAK1 mitogen-activated protein kinase kinase kinase is activated by autophosphorylation within its activation loop. J Biol Chem 2000; 275: 7359–7364.
Scholz R, Sidler CL, Thali RF, Winssinger N, Cheung PC, Neumann D . Autoactivation of transforming growth factor β-activated kinase 1 is a sequential bimolecular process. J Biol Chem 2010; 285: 25753–25766.
Kamisuki S, Mao Q, Abu-Elheiga L, Gu Z, Kugimiya A, Kwon Y et al. A small molecule that blocks fat synthesis by inhibiting the activation of SREBP. Chem Biol 2009; 16: 882–892.
Glass CK, Olefsky JM . Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab 2012; 15: 635–645.
Zhang HH, Halbleib M, Ahmad F, Manganiello VC, Greenberg AS . Tumor necrosis factor-α stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes 2002; 51: 2929–2935.
Fon Tacer K, Kuzman D, Seliskar M, Pompon D, Rozman D . TNF-α interferes with lipid homeostasis and activates acute and proatherogenic processes. Physiol Genomics 2007; 31: 216–227.
Oliner JD, Andresen JM, Hansen SK, Zhou SL, Tjian R . SREBP transcriptional activity is mediated through an interaction with the CREB-binding protein. Genes Dev 1996; 10: 2903–2911.
Osborne TF . Sterol regulatory element-binding proteins (SREBPs): key regulators of nutritional homeostasis and insulin action. J Biol Chem 2000; 275: 32379–32382.
Junttila MR, Li SP, Westermarck J . Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J 2008; 22: 954–965.
Kim SI, Kwak JH, Wang L, Choi ME . Protein phosphatase 2A is a negative regulator of transforming growth factor-β1-induced TAK1 activation in mesangial cells. J Biol Chem 2008; 283: 10753–10763.
Kajino T, Ren H, Iemura S, Natsume T, Stefansson B, Brautigan DL et al. Protein phosphatase 6 down-regulates TAK1 kinase activation in the IL-1 signaling pathway. J Biol Chem 2006; 281: 39891–39896.
Shimomura I, Hammer RE, Richardson JA, Ikemoto S, Bashmakov Y, Goldstein JL et al. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev 1998; 12: 3182–3194.
Nakayama H, Otabe S, Ueno T, Hirota N, Yuan X, Fukutani T et al. Transgenic mice expressing nuclear sterol regulatory element-binding protein 1c in adipose tissue exhibit liver histology similar to nonalcoholic steatohepatitis. Metabolism 2007; 56: 470–475.
Takahashi Y, Soejima Y, Fukusato T . Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 2012; 18: 2300–2308.
Campbell JS, Hughes SD, Gilbertson DG, Palmer TE, Holdren MS, Haran AC et al. Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc Natl Acad Sci USA 2005; 102: 3389–3394.
Heindryckx F, Colle I, Van Vlierberghe H . Experimental mouse models for hepatocellular carcinoma research. Int J Exp Pathol 2009; 90: 367–386.
Farazi PA, DePinho RA . Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer 2006; 6: 674–687.
Zhang L, Yang F, Yuan JH, Yuan SX, Zhou WP, Huo XS et al. Epigenetic activation of the MiR-200 family contributes to H19-mediated metastasis suppression in hepatocellular carcinoma. Carcinogenesis 2013; 34: 577–586.
Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol 2005; 6: 1087–1095.
Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic β cell-specific gene knock-outs using Cre recombinase. J Biol Chem 1999; 274: 305–315.
Pfeffer K, Matsuyama T, Kundig TM, Wakeham A, Kishihara K, Shahinian A et al. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 1993; 73: 457–467.
Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K . The kinase TAK1 can activate the NIK-IκB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 1999; 398: 252–256.
Toth JI, Datta S, Athanikar JN, Freedman LP, Osborne TF . Selective coactivator interactions in gene activation by SREBP-1a and -1c. Mol Cell Biol 2004; 24: 8288–8300.
Hua X, Nohturfft A, Goldstein JL, Brown MS . Sterol resistance in CHO cells traced to point mutation in SREBP cleavage-activating protein. Cell 1996; 87: 415–426.
Uemura N, Kajino T, Sanjo H, Sato S, Akira S, Matsumoto K et al. TAK1 is a component of the Epstein-Barr virus LMP1 complex and is essential for activation of JNK but not of NF-κB. J Biol Chem 2006; 281: 7863–7872.
Morioka S, Omori E, Kajino T, Kajino-Sakamoto R, Matsumoto K, Ninomiya-Tsuji J . TAK1 kinase determines TRAIL sensitivity by modulating reactive oxygen species and cIAP. Oncogene 2009; 28: 2257–2265.
Ninomiya-Tsuji J, Kajino T, Ono K, Ohtomo T, Matsumoto M, Shiina M et al. A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem 2003; 278: 18485–18490.
Smith JR, Osborne TF, Goldstein JL, Brown MS . Identification of nucleotides responsible for enhancer activity of sterol regulatory element in low density lipoprotein receptor gene. J Biol Chem 1990; 265: 2306–2310.
Ringseis R, Rauer C, Rothe S, Gessner DK, Schutz LM, Luci S et al. Sterol regulatory element-binding proteins are regulators of the NIS gene in thyroid cells. Mol Endocrinol 2013; 27: 781–800.
Acknowledgements
We thank NC State biological animal facility for technical support, Dr Akira for Tak1-loxed mice and Simmons, A. for critical reading. This work was supported by National Institutes of Health Grant GM068812 (to JNT).
Author contributions
SM, KS, EO, YI and JNT performed the experiments and analyzed the data. SM, KM and JNT designed the experiments and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Morioka, S., Sai, K., Omori, E. et al. TAK1 regulates hepatic lipid homeostasis through SREBP. Oncogene 35, 3829–3838 (2016). https://doi.org/10.1038/onc.2015.453
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.453
This article is cited by
-
Innate immune regulatory networks in hepatic lipid metabolism
Journal of Molecular Medicine (2019)
-
SREBP-regulated lipid metabolism: convergent physiology — divergent pathophysiology
Nature Reviews Endocrinology (2017)
-
Noncanonical cell death program independent of caspase activation cascade and necroptotic modules is elicited by loss of TGFβ-activated kinase 1
Scientific Reports (2017)