Abstract
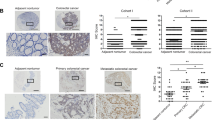
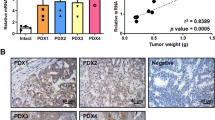
MACC1 (metastasis associated in colon cancer 1) is a prognostic biomarker for tumor progression, metastasis and survival of a variety of solid cancers including colorectal cancer (CRC). Here we aimed to identify the MACC1-induced transcriptome and key players mediating the MACC1-induced effects in CRC. We performed microarray analyses using CRC cells ectopically overexpressing MACC1. We identified more than 1300 genes at least twofold differentially expressed, including the gene SPON2 (Spondin 2) as 90-fold upregulated transcriptional target of MACC1. MACC1-dependent SPON2 expression regulation was validated on mRNA and protein levels in MACC1 high (endogenously or ectopically) and low (endogenously or by knockdown) expressing cells. Chromatin immunoprecipitation analysis demonstrated the binding of MACC1 to the gene promoter of SPON2. In cell culture, ectopic SPON2 overexpression induced cell viability, migration, invasion and colony formation in endogenously MACC1 and SPON2 low expressing cells, whereas SPON2 knockdown reduced proliferative, migratory and invasive abilities in CRC cells with high endogenous MACC1 and SPON2 expression. In intrasplenically transplanted NOD/SCID mice, metastasis induction was analyzed with control or SPON2-overexpressing CRC cells. Tumors with SPON2 overexpression induced liver metastasis (vs control animals without any metastases, P=0.0036). In CRC patients, SPON2 expression was determined in primary tumors (stages I–III), and survival time was analyzed by Kaplan–Meier method. CRC patients with high SPON2 expressing primary tumors demonstrated 8 months shorter metastasis-free survival (MFS) compared with patients with low SPON2 levels (P=0.053). Combining high levels of SPON2 and MACC1 improved the identification of high-risk patients with a 20-month shorter MFS vs patients with low biomarker expression. In summary, SPON2 is a transcriptional target of the metastasis gene MACC1. SPON2 induces cell motility in vitro and CRC metastasis in mice. In patients, SPON2 serves as prognostic indicator for CRC metastasis and survival, and might represent a promising target for therapeutic approaches.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ferlay J, Soerjomataram II, Dikshit R, Eser S, Mathers C, Rebelo M et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–E386.
Stein U, Schlag PM . Clinical, biological, and molecular aspects of metastasis in colorectal cancer. Recent Results Cancer Res 2007; 176: 61–80.
Stein U, Walther W, Arlt F, Schwabe H, Smith J, Fichtner I et al. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med 2009; 15: 59–67.
Nitsche U, Rosenberg R, Balmert A, Schuster T, Slotta-Huspenina J, Herrmann P et al. Integrative marker analysis allows risk assessment for metastasis in stage II colon cancer. Ann Surg 2012; 256: 763–777.
Schmid F, Burock S, Klockmeier K, Schlag PM, Stein U . SNPs in the coding region of the metastasis-inducing gene MACC1 and clinical outcome in colorectal cancer. Mol Cancer 2012; 11: 49.
Stein U, Burock S, Herrmann P, Wendler I, Niederstrasser M, Wernecke KW et al. Circulating MACC1 transcripts in colorectal cancer patient plasma predict metastasis and prognosis. PLoS One 2012; 7: e49249.
Ilm K, Kemmner W, Osterland M, Burock S, Koch G, Herrman P et al. High MACC1 expression in combination with mutated KRAS G13 indicates poor survival of colorectal cancer patients. Mol Cancer 2015; 14: 38.
Koelzer VH, Herrmann P, Zlobec I, Karamitopoulou E, Lugli A, Stein U . Heterogeneity analysis of metastasis associated in colon cancer 1 (MACC1) for survival prognosis of colorectal cancer patients: a retrospective cohort study. BMC Cancer 2015; 15: 160.
Stein U . MACC1 - a novel target for solid cancers. Expert Opin Ther Targets 2013; 17: 1039–1052.
Hagemann C, Fuchs S, Monoranu CM, Herrmann P, Smith J, Hohmann T et al. Impact of MACC1 on human malignant glioma progression and unfavorable patients’ prognosis. Neuro Oncol 2013; 15: 1696–1709.
Burock S, Herrmann P, Wendler I, Niederstrasser M, Wernecke KD, Stein U . Circulating MACC1 transcripts in gastric cancer patient plasma as diagnostic and prognostic biomarker. World J Gastroenterol 2015; 21: 333–341.
Stein U, Dahlmann M, Walther W . MACC1 – more than metastasis? Facts and predictions about a novel gene. J Mol Med 2010; 88: 11–18.
Juneja M, Ilm K, Schlag PM, Stein U . Promoter identification and transcriptional regulation of the metastasis gene MACC1 in colorectal cancer. Mol Oncol 2013; 7: 929–943.
Pichorner A, Sack U, Kobelt D, Kelch I, Arlt F, Smith J et al. In vivo imaging of colorectal cancer growth and metastasis by targeting MACC1 with shRNA in xenografted mice. Clin Exp Metastasis 2012; 29: 573–583.
Arlt F, Stein U . Colon cancer metastasis: MACC1 and Met as metastatic pacemakers. Int J Biochem Cell Biol 2009; 41: 2356–2359.
Stein U, Smith J, Walther W, Arlt F . MACC1 controls Met: what a difference an Sp1 site makes. Cell Cycle 2009; 8: 2467–2469.
Gao CF, Vande Woude GF . HGF/SF-Met signaling in tumor progression. Cell Res 2005; 15: 49–51.
Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G . Targeting MET in cancer: rationale and progress. Nat Rev Cancer 2012; 12: 89–103.
Manda R, Kohno T, Matsuno Y, Takenoshita S, Kuwano H, Yokota J . Identification of genes (SPON2 and C20orf2) differentially expressed between cancerous and noncancerous lung cells by mRNA differential display. Genomics 1999; 61: 5–14.
Carvalho B, Sillars-Hardebol AH, Postma C, Mongera S, Terhaar Sive Droste J, Obulkasim A et al. Colorectal adenoma to carcinoma progression is accompanied by changes in gene expression associated with ageing, chromosomal instability, and fatty acid metabolism. Cell Oncol (Dordr) 2012; 35: 53–63.
Benjamini Y, Hochberg Y . Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol 1995; 57: 289–300.
Wagstaff L, Kelwick R, Decock J, Edwards DR . The roles of ADAMTS metalloproteinases in tumorigenesis and metastasis. Front Biosci 2011; 16: 1861–1872.
Neufeld G, Sabag AD, Rabinovicz N, Kessler O . Semaphorins in angiogenesis and tumor progression. Cold Spring Harb Perspect Med 2012; 2: a006718.
Sakurai A, Doci CL, Gutkind JS . Semaphorin signaling in angiogenesis, lymphangiogenesis and cancer. Cell Res 2012; 22: 23–32.
Galimi F, Torti D, Sassi F, Isella C, Corà D, Gastaldi S et al. Genetic and expression analysis of MET, MACC1, and HGF in metastatic colorectal cancer: response to met inhibition in patient xenografts and pathologic correlations. Clin Cancer Res 2011; 17: 3146–3156.
Wang N, Xie JM, Zheng DY, Zuo Q, Liao WJ . [Establishment of BGC-823/pBaBb-puro-MACC1 gastric cancer cell line stably expressing MACC1 and its tumor-related gene expression profiles]. Nan Fang Yi Ke Da Xue Xue Bao 2012; 32: 312–316.
Jia W, Li H, He YW . The extracellular matrix protein mindin serves as an integrin ligand and is critical for inflammatory cell recruitment. Blood 2005; 106: 3854–3859.
Desgrosellier JS, Cheresh DA . Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 2010; 10: 9–22.
Li H, Oliver T, Jia W, He YW . Efficient dendritic cell priming of T lymphocytes depends on the extracellular matrix protein mindin. EMBO J 2006; 25: 4097–4107.
Ridley AJ . Rho GTPases and cell migration. J Cell Sci 2001; 114: 2713–2722.
Parry R, Schneider D, Hudson D, Parkes D, Xuan JA, Newton A et al. Identification of a novel prostate tumor target, mindin/RG-1, for antibody-based radiotherapy of prostate cancer. Cancer Res 2005; 65: 8397–8405.
Edwards S, Campbell C, Flohr P, Shipley J, Giddings I, Te-Poele R et al. Expression analysis onto microarrays of randomly selected cDNA clones highlights HOXB13 as a marker of human prostate cancer. Br J Cancer 2005; 92: 376–381.
Romanuik TL, Ueda T, Le N, Haile S, Yong TM, Thomson T et al. Novel biomarkers for prostate cancer including noncoding transcripts. Am J Pathol 2009; 175: 2264–2276.
Qian X, Li C, Pang B, Xue M, Wang J, Zhou J . Spondin-2 (SPON2), a more prostate-cancer-specific diagnostic biomarker. PLoS One 2012; 7: e37225.
Lucarelli G, Rutigliano M, Bettocchi C, Palazzo S, Vavallo A, Galleggiante V et al. Spondin-2, a secreted extracellular matrix protein, is a novel diagnostic biomarker for prostate cancer. J Urol 2013; 190: 2271–2277.
Simon I, Liu Y, Krall KL, Urban N, Wolfert RL, Kim NW et al. Evaluation of the novel serum markers B7-H4, Spondin 2, and DcR3 for diagnosis and early detection of ovarian cancer. Gynecol Oncol 2007; 106: 112–118.
Anderson GL, McIntosh M, Wu L, Barnett M, Goodman G, Thorpe JD et al. Assessing lead time of selected ovarian cancer biomarkers: a nested case-control study. J Natl Cancer Inst 2010; 102: 26–38.
Rajkumar T, Vijayalakshmi N, Gopal G, Sabitha K, Shirley S, Raja UM et al. Identification and validation of genes involved in gastric tumorigenesis. Cancer Cell Int 2010; 10: 45.
Zhang Q, Wang XQ, Wang J, Cui SJ, Lou XM, Yan B et al. Upregulation of spondin-2 predicts poor survival of colorectal carcinoma patients. Oncotarget 2015; 6: 15095–15110.
Huang, da W, Sherman BT, Lempicki RA . Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4: 44–57.
Huang, da W, Sherman BT, Lempicki RA . Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 2009; 37: 1–13.
Stein U, Arlt F, Smith J, Sack U, Herrmann P, Walther W et al. Intervening in b-catenin signaling by sulindac inhibits S100A4-dependent colon cancer metastasis. Neoplasia 2011; 13: 131–144.
Acknowledgements
We are thankful to Wolfgang Kemmner and his laboratory for the help with the microarray experiments. We thank Maria Stecklum and Nadine Bäsler for support with the in vivo bioluminescence imaging, and Jutta Aumann for assistance on expression analyses. We thank Manisha Juneja and Katharina Ilm for help with the combinatorial biomarker analysis in the CRC patients. We are grateful to Markus Niederstrasser for statistical advice. This work was supported by the German Cancer Consortium (DKTK).
Author contributions
FS and US conceived and designed the experiments and wrote the manuscript. FS and JS performed the in vitro experiments. FS, QW, MRH and MAA-N analyzed the microarray data. FS, ML and IF designed and performed the in vivo studies. MD, DK and WW evaluated the experimental data and contributed to the revised version of the manuscript. PMS evaluated the clinical data. All authors have read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Schmid, F., Wang, Q., Huska, M. et al. SPON2, a newly identified target gene of MACC1, drives colorectal cancer metastasis in mice and is prognostic for colorectal cancer patient survival. Oncogene 35, 5942–5952 (2016). https://doi.org/10.1038/onc.2015.451
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.451
This article is cited by
-
Tumor cell-derived SPON2 promotes M2-polarized tumor-associated macrophage infiltration and cancer progression by activating PYK2 in CRC
Journal of Experimental & Clinical Cancer Research (2021)
-
Screening of radiotracer for diagnosis of colorectal cancer liver metastasis based on MACC1-SPON2
Abdominal Radiology (2021)
-
Quantitative proteomic analysis of prostate tissue specimens identifies deregulated protein complexes in primary prostate cancer
Clinical Proteomics (2019)
-
DBC1 regulates Wnt/β-catenin-mediated expression of MACC1, a key regulator of cancer progression, in colon cancer
Cell Death & Disease (2018)
-
The extracellular matrix protein mindin attenuates colon cancer progression by blocking angiogenesis via Egr-1-mediated regulation
Oncogene (2018)