Abstract
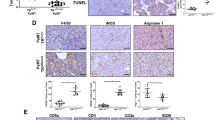
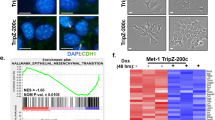
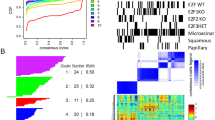
The Rb-E2F axis is an important pathway involved in cell-cycle control that is deregulated in a number of cancers. E2f transcription factors have distinct roles in the control of cell proliferation, cell survival and differentiation in a variety of tissues. We have previously shown that E2fs are important downstream targets of a CSF-1 signaling cascade involved in myeloid development. In cancer, tumor-associated macrophages (TAMs) are recruited to the tumor stroma in response to cytokines secreted by tumor cells, and are believed to facilitate tumor cell invasion and metastasis. Using the MMTV-Polyoma Middle T antigen (PyMT) mouse model of human ductal carcinoma, we show that the specific ablation of E2f3 in TAMs, but not in tumor epithelial cells, attenuates lung metastasis without affecting primary tumor growth. Histological analysis and gene expression profiling suggest that E2f3 does not impact the proliferation or survival of TAMs, but rather controls a novel gene expression signature associated with cytoskeleton rearrangements, cell migration and adhesion. This E2f3 TAM gene expression signature was sufficient to predict cancer recurrence and overall survival of estrogen receptor (ER)-positive breast cancer patients. Interestingly, we find that E2f3b but not E2f3a levels are elevated in TAMs from PyMT mammary glands relative to controls, suggesting a differential role for these isoforms in metastasis. In summary, these findings identify E2f3 as a key transcription factor in TAMs, which influences the tumor microenvironment and tumor cell metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
O'Shaughnessy J . Extending survival with chemotherapy in metastatic breast cancer. Oncologist 2005; 10: 20–29.
Chiang A, Massagué J . Molecular basis of metastasis. N Engl J Med 2008; 359: 2814–2823.
Chaffer CL, Weinberg RA . A perspective on cancer cell metastasis. Science 2011; 331: 1559–1564.
Joyce J, Pollard J . Microenvironmental regulation of metastasis. Nat Rev Cancer 2009; 9: 239–252.
Condeelis J, Pollard J . Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006; 124: 263–266.
Kim BG, Li C, Qiao W, Mamura M, Kasprzak B, Kasperczak B et al. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature 2006; 441: 1015–1019.
DeNardo DG, Johansson M, Coussens LM . Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev 2008; 27: 11–18.
Bronisz A, Godlewski J, Wallace JA, Merchant AS, Nowicki MO, Mathsyaraja H et al. Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. Nat Cell Biol 2012; 14: 159–167.
Orimo A, Gupta P, Sgroi D, Arenzana-Seisdedos F, Delaunay T, Naeem R et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005; 121: 335–348.
Talmadge J, Donkor M, Scholar E . Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev 2007; 26: 373–400.
Leek R, Lewis C, Whitehouse R, Greenall M, Clarke J, Harris A . Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 1996; 56: 4625–4629.
Allavena P, Sica A, Solinas G, Porta C, Mantovani A . The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol 2008; 66: 1–9.
Diel I, Solomayer E, Bastert G . Bisphosphonates and the prevention of metastasis: first evidences from preclinical and clinical studies. Cancer 2000; 88: 3080–3088.
Mundy G, Yoneda T . Bisphosphonates as anticancer drugs. N Engl J Med 1998; 339: 398–400.
Nasser MW, Qamri Z, Deol YS, Ravi J, Powell CA, Trikha P et al. S100A7 enhances mammary tumorigenesis through upregulation of inflammatory pathways. Cancer Res 2011; 72: 604–615.
Wyckoff J, Wang W, Lin E, Wang Y, Pixley F, Stanley E et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res 2004; 64: 7022–7029.
Lin EY, Nguyen AV, Russell RG, Pollard JW . Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 2001; 193: 727–740.
Kacinski B . CSF-1 and its receptor in ovarian, endometrial and breast cancer. Ann Med 1995; 27: 79–85.
Trikha P, Sharma N, Opavsky R, Reyes A, Pena C, Ostrowski MC et al. E2f1-3 are critical for myeloid development. J Biol Chem 2011; 286: 4783–4795.
Bosco EE, Knudsen ES . RB in breast cancer: at the crossroads of tumorigenesis and treatment. Cell Cycle 2007; 6: 667–671.
Jiang Z, Deng T, Jones R, Li H, Herschkowitz JI, Liu JC et al. Rb deletion in mouse mammary progenitors induces luminal-B or basal-like/EMT tumor subtypes depending on p53 status. J Clin Invest 2010; 120: 3296–3309.
Nevins JR . The Rb/E2F pathway and cancer. Hum Mol Genet 2001; 10: 699–703.
Foster C, Falconer A, Dodson A, Norman A, Dennis N, Fletcher et al. Transcription factor E2F3 overexpressed in prostate cancer independently predicts clinical outcome. Oncogene 2004; 23: 5871–5879.
Cooper C, Nicholson A, Foster C, Dodson A, Edwards S, Fletcher et al. Nuclear overexpression of the E2F3 transcription factor in human lung cancer. Lung Cancer 2006; 54: 155–162.
Olsson A, Feber A, Edwards S, Te Poele R, Giddings I, Merson S et al. Role of E2F3 expression in modulating cellular proliferation rate in human bladder and prostate cancer cells. Oncogene 2007; 26: 1028–1037.
De Meyer T, Bijsmans IT, Van de Vijver KK, Bekaert S, Oosting J, Van Criekinge W et al. E2Fs mediate a fundamental cell-cycle deregulation in high-grade serous ovarian carcinomas. J Pathol 2009; 217: 14–20.
Lujambio A, Calin G, Villanueva A, Ropero S, Sánchez-Céspedes M, Blanco D et al. A microRNA DNA methylation signature for human cancer metastasis. Proc Natl Acad Sci USA 2008; 105: 13556–13561.
Van Nguyen A, Pollard JW . Colony stimulating factor-1 is required to recruit macrophages into the mammary gland to facilitate mammary ductal outgrowth. Dev Biol 2002; 247: 11–25.
Mootha V, Lindgren C, Eriksson K, Subramanian A, Sihag S, Lehar J et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003; 34: 267–273.
Hurley PJ, Marchionni L, Simons BW, Ross AE, Peskoe SB, Miller RM et al. Secreted protein, acidic and rich in cysteine-like 1 (SPARCL1) is down regulated in aggressive prostate cancers and is prognostic for poor clinical outcome. Proc Natl Acad Sci USA 2012; 109: 14977–14982.
Yue W, Sun Q, Landreneau R, Wu C, Siegfried JM, Yu J et al. Fibulin-5 suppresses lung cancer invasion by inhibiting matrix metalloproteinase-7 expression. Cancer Res 2009; 69: 6339–6346.
Reynolds LE, Watson AR, Baker M, Jones TA, D'Amico G, Robinson SD et al. Tumour angiogenesis is reduced in the Tc1 mouse model of Down's syndrome. Nature 2010; 465: 813–817.
Leone G, Nuckolls F, Ishida S, Adams M, Sears R, Jakoi L et al. Identification of a novel E2F3 product suggests a mechanism for determining specificity of repression by Rb proteins. Mol Cell Biol 2000; 20: 3626–3632.
Adams M, Sears R, Nuckolls F, Leone G, Nevins J . Complex transcriptional regulatory mechanisms control expression of the E2F3 locus. Mol Cell Biol 2000; 20: 3633–3639.
Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med 2008; 14: 518–527.
Chen H, Tsai S, Leone G . Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer 2009; 9: 785–797.
Wu L, de Bruin A, Wang H, Simmons T, Cleghorn W, Goldenberg LE et al. Selective roles of E2Fs for ErbB2- and Myc-mediated mammary tumorigenesis. Oncogene 2013.
Ziebold U, Lee EY, Bronson RT, Lees JA . E2F3 loss has opposing effects on different pRB-deficient tumors, resulting in suppression of pituitary tumors but metastasis of medullary thyroid carcinomas. Mol Cell Biol 2003; 23: 6542–6552.
Ceol CJ, Horvitz HR . dpl-1 DP and efl-1 E2F act with lin-35 Rb to antagonize Ras signaling in C. elegans vulval development. Mol Cell 2001; 7: 461–473.
Myers TR, Greenwald I . lin-35 Rb acts in the major hypodermis to oppose ras-mediated vulval induction in C. elegans. Dev Cell 2005; 8: 117–123.
Blanchet E, Annicotte JS, Lagarrigue S, Aguilar V, Clapé C, Chavey C et al. E2F transcription factor-1 regulates oxidative metabolism. Nat Cell Biol 2011; 13: 1146–1152.
Gauthier ML, Berman HK, Miller C, Kozakeiwicz K, Chew K, Moore D et al. Abrogated response to cellular stress identifies DCIS associated with subsequent tumor events and defines basal-like breast tumors. Cancer Cell 2007; 12: 479–491.
Bornstein P, Sage EH . Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol 2002; 14: 608–616.
Brekken RA, Puolakkainen P, Graves DC, Workman G, Lubkin SR, Sage EH . Enhanced growth of tumors in SPARC null mice is associated with changes in the ECM. J Clin Invest 2003; 111: 487–495.
Sangaletti S, Stoppacciaro A, Guiducci C, Torrisi MR, Colombo MP . Leukocyte, rather than tumor-produced SPARC, determines stroma and collagen type IV deposition in mammary carcinoma. J Exp Med 2003; 198: 1475–1485.
Stetler-Stevenson WG, Aznavoorian S, Liotta LA . Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol 1993; 9: 541–573.
van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002; 347: 1999–2009.
Pawitan Y, Bjöhle J, Amler L, Borg AL, Egyhazi S, Hall P et al. Gene expression profiling spares early breast cancer patients from adjuvant therapy: derived and validated in two population-based cohorts. Breast Cancer Res 2005; 7: R953–R964.
Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet 2005; 365: 671–679.
Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I . Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 1999; 8: 265–277.
Acknowledgements
We thank Bryan Mcelwain for technical assistance with flow cytometry. This work was funded by NIH grants R01CA121275, R01CA098956 and P01CA097189 MC and GL to GL. PT and NS are recipients of the T32 fellowship (CA 106196-06).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Trikha, P., Sharma, N., Pena, C. et al. E2f3 in tumor macrophages promotes lung metastasis. Oncogene 35, 3636–3646 (2016). https://doi.org/10.1038/onc.2015.429
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.429
This article is cited by
-
Tumor necroptosis-mediated shedding of cell surface proteins promotes metastasis of breast cancer by suppressing anti-tumor immunity
Breast Cancer Research (2023)
-
ZIM3 activation of CCL25 expression in pulmonary metastatic nodules of osteosarcoma recruits M2 macrophages to promote metastatic growth
Cancer Immunology, Immunotherapy (2023)
-
Upregulation of long noncoding RNA linc02544 and its association with overall survival rate and the influence on cell proliferation and migration in lung squamous cell carcinoma
Discover Oncology (2022)
-
ZBP1 not RIPK1 mediates tumor necroptosis in breast cancer
Nature Communications (2021)
-
A novel LncRNA transcript, RBAT1, accelerates tumorigenesis through interacting with HNRNPL and cis-activating E2F3
Molecular Cancer (2020)