Abstract
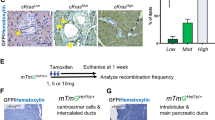
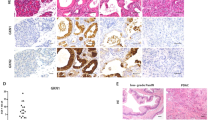
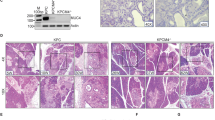
Intraductal papillary mucinous neoplasm (IPMN), the most common pancreatic cystic neoplasm, is known to progress to invasive ductal adenocarcinoma. IPMNs commonly harbor activating somatic mutations in GNAS and KRAS, primarily GNASR201H and KRASG12D. GNAS encodes the stimulatory G-protein α subunit (Gsα) that mediates a stimulatory signal to adenylyl cyclase to produce cyclic adenosine monophosphate (cAMP), subsequently activating cAMP-dependent protein kinase A. The GNASR201H mutation results in constitutive activation of Gsα. To study the potential role of GNAS in pancreatic tumorigenesis in vivo, we generated lines of transgenic mice in which the transgene consisted of Lox-STOP-Lox (LSL)-GNASR201H under the control of the CAG promoter (Tg(CAG-LSL-GNAS)). These mice were crossed with pancreatic transcription factor 1a (Ptf1a)-Cre mice (Ptf1aCre/+), generating Tg(CAG-LSL-GNAS);Ptf1aCre/+ mice. This mouse line showed elevated cAMP levels, small dilated tubular complex formation, loss of acinar cells and fibrosis in the pancreas; however, no macroscopic tumorigenesis was apparent by 2 months of age. We then crossed Tg(CAG-LSL-GNAS);Ptf1aCre/+ mice with LSL-KrasG12D mice, generating Tg(CAG-LSL-GNAS);LSL-KrasG12D;Ptf1aCre/+ mice. We used these mice to investigate a possible cooperative effect of GNASR201H and KrasG12D in pancreatic tumorigenesis. Within 5 weeks, Tg(CAG-LSL-GNAS);LSL-KrasG12D;Ptf1aCre/+ mice developed a cystic tumor consisting of marked dilated ducts lined with papillary dysplastic epithelia in the pancreas, which closely mimicked the human IPMN. Our data strongly suggest that activating mutations in GNAS and Kras cooperatively promote murine pancreatic tumorigenesis, which recapitulates IPMN. Our mouse model may serve as a unique in vivo platform to find biomarkers and effective drugs for diseases associated with GNAS mutations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Adsay NV, Fukushima N, Furukawa T, Hruban RH, Klimstra DS, Kloppel G et alIntraductal neoplasms of the pancreas. In: Bosman FT, Hruban RH, Carneiro F et al. (eds). WHO Classification of Tumours of the Digestive System, 4th edn. IARC: Lyon, 2010, pp 304–313.
Furukawa T, Hatori T, Fujita I, Yamamoto M, Kobayashi M, Ohike N et al. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut 2011; 60: 509–516.
Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 2011; 3: 92ra66.
Furukawa T, Kuboki Y, Tanji E, Yoshida S, Hatori T, Yamamoto M et al. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep 2011; 1: 161.
Matthaei H, Wu J, Dal Molin M, Shi C, Perner S, Kristiansen G et al. GNAS sequencing identifies IPMN-specific mutations in a subgroup of diminutive pancreatic cysts referred to as "incipient IPMNs”. Am J Surg Pathol 2014; 38: 360–363.
Dhanasekaran DN . Transducing the signals: a G protein takes a new identity. Sci STKE 2006; 2006: pe31.
Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L . GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature 1989; 340: 692–696.
O'Sullivan C, Barton CM, Staddon SL, Brown CL, Lemoine NR . Activating point mutations of the gsp oncogene in human thyroid adenomas. Mol Carcinog 1991; 4: 345–349.
Fragoso MC, Latronico AC, Carvalho FM, Zerbini MC, Marcondes JA, Araujo LM et al. Activating mutation of the stimulatory G protein (gsp) as a putative cause of ovarian and testicular human stromal Leydig cell tumors. J Clin Endocrinol Metab 1998; 83: 2074–2078.
Kalfa N, Lumbroso S, Boulle N, Guiter J, Soustelle L, Costa P et al. Activating mutations of Gsalpha in kidney cancer. J Urol 2006; 176: 891–895.
Delaney D, Diss TC, Presneau N, Hing S, Berisha F, Idowu BD et al. GNAS1 mutations occur more commonly than previously thought in intramuscular myxoma. Mod Pathol 2009; 22: 718–724.
Yamada M, Sekine S, Ogawa R, Taniguchi H, Kushima R, Tsuda H et al. Frequent activating GNAS mutations in villous adenoma of the colorectum. J Pathol 2012; 228: 113–118.
Nishikawa G, Sekine S, Ogawa R, Matsubara A, Mori T, Taniguchi H et al. Frequent GNAS mutations in low-grade appendiceal mucinous neoplasms. Br J Cancer 2013; 108: 951–958.
Matsubara A, Sekine S, Kushima R, Ogawa R, Taniguchi H, Tsuda H et al. Frequent GNAS and KRAS mutations in pyloric gland adenoma of the stomach and duodenum. J Pathol 2013; 229: 579–587.
Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM . Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med 1991; 325: 1688–1695.
Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV . The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet 2002; 32: 128–134.
Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003; 4: 437–450.
Hata T, Sakata N, Okada T, Aoki T, Motoi F, Katayose Y et al. Dilated papilla with mucin extrusion is a potential predictor of acute pancreatitis associated with intraductal papillary mucinous neoplasms of pancreas. Pancreatology 2013; 13: 615–620.
Kuboki Y, Shimizu K, Hatori T, Yamamoto M, Shibata N, Shiratori K et al. Molecular biomarkers for progression of intraductal papillary mucinous neoplasm of the pancreas. Pancreas 2015; 44: 227–235.
Komatsu H, Tanji E, Sakata N, Aoki T, Motoi F, Naitoh T et al. A GNAS mutation found in pancreatic intraductal papillary mucinous neoplasms induces drastic alterations of gene expression profiles with upregulation of mucin genes. PloS One 2014; 9: e87875.
Taniwaki T, Haruna K, Nakamura H, Sekimoto T, Oike Y, Imaizumi T et al. Characterization of an exchangeable gene trap using pU-17 carrying a stop codon-beta geo cassette. Dev Growth Differ 2005; 47: 163–172.
Semba K, Araki K, Matsumoto K, Suda H, Ando T, Sei A et al. Ectopic expression of Ptf1a induces spinal defects, urogenital defects, and anorectal malformations in Danforth's short tail mice. PloS Genet 2013; 9: e1003204.
Acknowledgements
We are grateful to Dr David A Tuveson (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, USA) for providing LSL-KrasG12D mouse, Dr Takeshi Tanaka and Dr Emiko Hayama (Department of Pediatric Cardiology, Tokyo Women’s Medical University) for their work preparing the anti-Gsα antibody and Ms Yuko Fukuchi and Ms Michiyo Nakata (Institute of Resource Development and Analysis, Kumamoto University) for their technical assistance. This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas, Ministry of Education, Culture, Sports, Science and Technology (26110003) to MO and a Grant-in-Aid for Challenging Exploratory Research, Japan Society for the Promotion of Science (24659167) to TF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Taki, K., Ohmuraya, M., Tanji, E. et al. GNASR201H and KrasG12D cooperate to promote murine pancreatic tumorigenesis recapitulating human intraductal papillary mucinous neoplasm. Oncogene 35, 2407–2412 (2016). https://doi.org/10.1038/onc.2015.294
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.294
This article is cited by
-
Mutant GNAS limits tumor aggressiveness in established pancreatic cancer via antagonizing the KRAS-pathway
Journal of Gastroenterology (2022)
-
Cathepsin B and D deficiency in the mouse pancreas induces impaired autophagy and chronic pancreatitis
Scientific Reports (2021)
-
Models of pancreatic ductal adenocarcinoma
Cancer and Metastasis Reviews (2021)
-
How does intestinal-type intraductal papillary mucinous neoplasm emerge? CDX2 plays a critical role in the process of intestinal differentiation and progression
Virchows Archiv (2020)
-
Inhibition of 15-PGDH causes Kras-driven tumor expansion through prostaglandin E2-ALDH1 signaling in the pancreas
Oncogene (2019)