Abstract
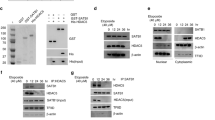
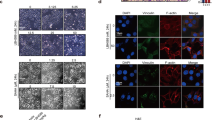
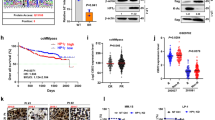
Phosphatase and tensin homologue deleted on chromosome 10 (PTEN), an important tumour-suppressor gene, is mutated, downregulated or dysfunctional in many tumours. The phosphatase activity of PTEN depends on membrane translocation (activation). As promising anti-cancer agents, histone deacetylase (HDAC) inhibitors, particularly trichostatin A (TSA), can promote PTEN membrane translocation, but the underlying mechanism remains unknown. In this study, we revealed that non-selective HDAC inhibitors, namely, TSA or suberoylanilide hydroxamic acid (SAHA), induced PTEN membrane translocation through PTEN acetylation at K163 by inhibiting HDAC6. K163 acetylation inhibited the interaction of the PTEN C-tail with the remaining part of PTEN, resulting in PTEN membrane translocation. Overexpression of wild-type PTEN, but not K163-mutated PTEN, facilitated the inhibition of cell proliferation, migration and invasion, as well as xenograft tumour growth, induced by SAHA or tubastatin A, an HDAC6-specific inhibitor. These results indicated that PTEN activation by inhibiting HDAC6 significantly contributed to tumour inhibition. Therefore, non-selective HDAC or HDAC6-specific inhibitors may be more clinically suitable to treat tumours without PTEN mutations or deletions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 1997; 15: 356–362.
Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997; 275: 1943–1947.
Li DM, Sun H . TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res 1997; 57: 2124–2129.
Tashiro H, Blazes MS, Wu R, Cho KR, Bose S, Wang SI et al. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res 1997; 57: 3935–3940.
Tsao H, Zhang X, Benoit E, Haluska FG . Identification of PTEN/MMAC1 alterations in uncultured melanomas and melanoma cell lines. Oncogene 1998; 16: 3397–3402.
Squarize CH, Castilho RM, Abrahao AC, Molinolo A, Lingen MW, Gutkind JS . PTEN deficiency contributes to the development and progression of head and neck cancer. Neoplasia 2013; 15: 461–471.
Maehama T, Dixon JE . The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 1998; 273: 13375–13378.
Li DM, Sun H . PTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cells. Proc Natl Acad Sci USA 1998; 95: 15406–15411.
Sun H, Lesche R, Li DM, Liliental J, Zhang H, Gao J et al. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci USA 1999; 96: 6199–6204.
Leslie NR, Yang X, Downes CP, Weijer CJ . The regulation of cell migration by PTEN. Biochem Soc Trans 2005; 33: 1507–1508.
Wen S, Stolarov J, Myers MP, Su JD, Wigler MH, Tonks NK et al. PTEN controls tumor-induced angiogenesis. Proc Natl Acad Sci USA 2001; 98: 4622–4627.
Vazquez F, Matsuoka S, Sellers WR, Yanagida T, Ueda M, Devreotes PN . Tumor suppressor PTEN acts through dynamic interaction with the plasma membrane. Proc Natl Acad Sci USA 2006; 103: 3633–3638.
Iijima M, Huang YE, Luo HR, Vazquez F, Devreotes PN . Novel mechanism of PTEN regulation by its phosphatidylinositol 4,5-bisphosphate binding motif is critical for chemotaxis. J Biol Chem 2004; 279: 16606–16613.
Denning G, Jean-Joseph B, Prince C, Durden DL, Vogt PK . A short N-terminal sequence of PTEN controls cytoplasmic localization and is required for suppression of cell growth. Oncogene 2007; 26: 3930–3940.
Walker SM, Leslie NR, Perera NM, Batty IH, Downes CP . The tumour-suppressor function of PTEN requires an N-terminal lipid-binding motif. Biochem J 2004; 379: 301–307.
Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y et al. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 1999; 99: 323–334.
Das S, Dixon JE, Cho W . Membrane-binding and activation mechanism of PTEN. Proc Natl Acad Sci USA 2003; 100: 7491–7496.
Lumb CN, Sansom MS . Defining the membrane-associated state of the PTEN tumor suppressor protein. Biophys J 2013; 104: 613–621.
Meuillet EJ, Mahadevan D, Berggren M, Coon A, Powis G . Thioredoxin-1 binds to the C2 domain of PTEN inhibiting PTEN's lipid phosphatase activity and membrane binding: a mechanism for the functional loss of PTEN's tumor suppressor activity. Arch Biochem Biophys 2004; 429: 123–133.
Huang J, Yan J, Zhang J, Zhu S, Wang Y, Shi T et al. SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane. Nat Commun 2012; 3: 911.
Rahdar M, Inoue T, Meyer T, Zhang J, Vazquez F, Devreotes PN . A phosphorylation-dependent intramolecular interaction regulates the membrane association and activity of the tumor suppressor PTEN. Proc Natl Acad Sci USA 2009; 106: 480–485.
Bolden JE, Peart MJ, Johnstone RW . Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 2006; 5: 769–784.
Okumura K, Mendoza M, Bachoo RM, DePinho RA, Cavenee WK, Furnari FB . PCAF modulates PTEN activity. J Biol Chem 2006; 281: 26562–26568.
Dekker FJ . Histone acetyl transferases as emerging drug targets. Drug Discovery Today 2009; 14: 942–948.
Witt O, Deubzer HE, Milde T, Oehme I . HDAC family: What are the cancer relevant targets? Cancer Lett 2009; 277: 8–21.
Zhang Y, Kwon S, Yamaguchi T, Cubizolles F, Rousseaux S, Kneissel M et al. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol Cell Biol 2008; 28: 1688–1701.
Lee YS, Lim KH, Guo X, Kawaguchi Y, Gao Y, Barrientos T et al. The cytoplasmic deacetylase HDAC6 is required for efficient oncogenic tumorigenesis. Cancer Res 2008; 68: 7561–7569.
Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL . Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci USA 2003; 100: 4389–4394.
Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D et al. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J 2002; 21: 6820–6831.
Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV et al. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell 2005; 18: 601–607.
Saji S, Kawakami M, Hayashi S, Yoshida N, Hirose M, Horiguchi S et al. Significance of HDAC6 regulation via estrogen signaling for cell motility and prognosis in estrogen receptor-positive breast cancer. Oncogene 2005; 24: 4531–4539.
Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A et al. HDAC6 is a microtubule-associated deacetylase. Nature 2002; 417: 455–458.
Altonsy MO, Habib TN, Andrews SC . Diallyl disulfide-induced apoptosis in a breast-cancer cell line (MCF-7) may be caused by inhibition of histone deacetylation. Nutr Cancer 2012; 64: 1251–1260.
Butler LM, Agus DB, Scher HI, Higgins B, Rose A, Cordon-Cardo C et al. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res 2000; 60: 5165–5170.
Kim HR, Kim EJ, Yang SH, Jeong ET, Park C, Lee JH et al. Trichostatin A induces apoptosis in lung cancer cells via simultaneous activation of the death receptor-mediated and mitochondrial pathway? Exp Mol Med 2006; 38: 616–624.
Herold C, Ganslmayer M, Ocker M, Hermann M, Geerts A, Hahn EG et al. The histone-deacetylase inhibitor Trichostatin A blocks proliferation and triggers apoptotic programs in hepatoma cells. J Hepatol 2002; 36: 233–240.
Donadelli M, Costanzo C, Faggioli L, Scupoli MT, Moore PS, Bassi C et al. Trichostatin A, an inhibitor of histone deacetylases, strongly suppresses growth of pancreatic adenocarcinoma cells. Mol Carcinog 2003; 38: 59–69.
Duan H, Heckman CA, Boxer LM . Histone deacetylase inhibitors down-regulate bcl-2 expression and induce apoptosis in t(14;18) lymphomas. Mol Cell Biol 2005; 25: 1608–1619.
Duvic M, Vu J . Vorinostat: a new oral histone deacetylase inhibitor approved for cutaneous T-cell lymphoma. Expert Opin Investig Drugs 2007; 16: 1111–1120.
Mei S, Ho AD, Mahlknecht U . Role of histone deacetylase inhibitors in the treatment of cancer (Review). Int J Oncol 2004; 25: 1509–1519.
Gan YH, Zhang S . PTEN/AKT pathway involved in histone deacetylases inhibitor induced cell growth inhibition and apoptosis of oral squamous cell carcinoma cells. Oral Oncol 2009; 45: e150–e154.
Kou XX, Hao T, Meng Z, Zhou YH, Gan YH . Acetylated Sp1 inhibits PTEN expression through binding to PTEN core promoter and recruitment of HDAC1 and promotes cancer cell migration and invasion. Carcinogenesis 2013; 34: 58–67.
Georgescu MM, Kirsch KH, Kaloudis P, Yang H, Pavletich NP, Hanafusa H . Stabilization and productive positioning roles of the C2 domain of PTEN tumor suppressor. Cancer Res 2000; 60: 7033–7038.
Tang Y, Zhao W, Chen Y, Zhao Y, Gu W . Acetylation is indispensable for p53 activation. Cell 2008; 133: 612–626.
Dallavalle S, Pisano C, Zunino F . Development and therapeutic impact of HDAC6-selective inhibitors. Biochem Pharmacol 2012; 84: 756–765.
Chen CS, Weng SC, Tseng PH, Lin HP . Histone acetylation-independent effect of histone deacetylase inhibitors on Akt through the reshuffling of protein phosphatase 1 complexes. J Biol Chem 2005; 280: 38879–38887.
Fischle W, Emiliani S, Hendzel MJ, Nagase T, Nomura N, Voelter W et al. A new family of human histone deacetylases related to Saccharomyces cerevisiae HDA1p. J Biol Chem 1999; 274: 11713–11720.
Oehme I, Linke JP, Bock BC, Milde T, Lodrini M, Hartenstein B et al. Histone deacetylase 10 promotes autophagy-mediated cell survival. Proc Natl Acad Sci USA 2013; 110: E2592–E2601.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81472764 and 81271173) and China International Science and Technology Cooperation (Grant No. 2013DFB30360).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Meng, Z., Jia, LF. & Gan, YH. PTEN activation through K163 acetylation by inhibiting HDAC6 contributes to tumour inhibition. Oncogene 35, 2333–2344 (2016). https://doi.org/10.1038/onc.2015.293
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.293
This article is cited by
-
Phospho PTEN mediated dephosphorylation of mitotic kinase PLK1 and Aurora Kinase A prevents aneuploidy and preserves genomic stability
Medical Oncology (2023)
-
HDAC6: A unique HDAC family member as a cancer target
Cellular Oncology (2022)
-
Upregulation of ERK-EGR1-heparanase axis by HDAC inhibitors provides targets for rational therapeutic intervention in synovial sarcoma
Journal of Experimental & Clinical Cancer Research (2021)
-
Selective HDAC6 inhibitors improve anti-PD-1 immune checkpoint blockade therapy by decreasing the anti-inflammatory phenotype of macrophages and down-regulation of immunosuppressive proteins in tumor cells
Scientific Reports (2019)
-
HDAC6 inhibitor TST strengthens the antiproliferative effects of PI3K/mTOR inhibitor BEZ235 in breast cancer cells via suppressing RTK activation
Cell Death & Disease (2018)