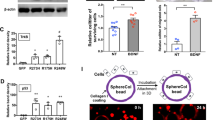
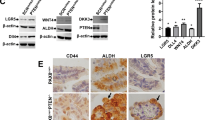
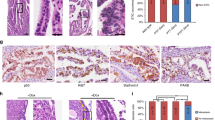
Abstract
Accumulating evidence indicates that ovarian high-grade serous carcinoma (HGSC) originates from fallopian tube secretory epithelial cells (FTSECs). However, the molecular mechanisms underlying the initiation and progression of HGSC derived from FTSECs remains unclear. In this study, we found that the Hippo/Yes-associated protein (YAP) signaling pathway has a critical role in the initiation and progression of fallopian tube and ovarian HGSC. Importantly, YAP was overexpressed in inflammatory and cancerous fallopian tube tissues. Further, overexpression of wild-type YAP, or constitutively active YAP in immortalized FTSECs, induced cell proliferation, migration, colony formation and tumorigenesis. Moreover, the Hippo/YAP and the fibroblast growth factor (FGF) signaling pathways formed an autocrine/paracrine-positive feedback loop to drive the progression of the FTSEC-derived HGSC. Evidence in this study strongly suggests that combined therapy with inhibitors of YAP (such as verteporfin) and FGF receptors (such as BGJ398) can provide a novel therapeutic strategy to treat fallopian tube and ovarian HGSC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D . Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90.
Seidman JD, Horkayne-Szakaly I, Haiba M, Boice CR, Kurman RJ, Ronnett BM . The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol 2004; 23: 41–44.
Selvaggi SM . Tumors of the ovary, maldeveloped gonads, fallopian tube, and broad ligament. Arch Pathol Lab Med 2000; 124: 477.
Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC . Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev 2001; 22: 255–288.
Katabuchi H, Okamura H . Cell biology of human ovarian surface epithelial cells and ovarian carcinogenesis. Med Electron Microsc 2003; 36: 74–86.
Casey MJ, Bewtra C, Hoehne LL, Tatpati AD, Lynch HT, Watson P . Histology of prophylactically removed ovaries from BRCA1 and BRCA2 mutation carriers compared with noncarriers in hereditary breast ovarian cancer syndrome kindreds. Gynecol Oncol 2000; 78: 278–287.
Barakat RR, Federici MG, Saigo PE, Robson ME, Offit K, Boyd J . Absence of premalignant histologic, molecular, or cell biologic alterations in prophylactic oophorectomy specimens from BRCA1 heterozygotes. Cancer 2000; 89: 383–390.
Berek JS, Crum C, Friedlander M . Cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet 2012; 119: S118–S129.
Kurman RJ, Shih IeM . Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer—shifting the paradigm. Hum Pathol 2011; 42: 918–931.
Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol 2007; 31: 161–169.
Goodman MT, Shvetsov YB . Incidence of ovarian, peritoneal, and fallopian tube carcinomas in the United States, 1995-2004. Cancer Epidemiol Biomarkers Prev 2009; 18: 132–139.
Johnson R, Halder G . The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov 2014; 13: 63–79.
Pan D . The Hippo signaling pathway in development and cancer. Dev Cell 2010; 19: 491–505.
Huang J, Wu S, Barrera J, Matthews K, Pan D . The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 2005; 122: 421–434.
Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber D et al. Salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 2002; 110: 467–478.
Yu FX, Guan KL . The Hippo pathway: regulators and regulations. Genes Dev 2013; 27: 355–371.
Zhang X, George J, Deb S, Degoutin JL, Takano EA, Fox SB et al. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene 2011; 30: 2810–2822.
Hall CA, Wang R, Miao J, Oliva E, Shen X, Wheeler T et al. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res 2010; 70: 8517–8525.
Fu D, Lv X, Hua G, He C, Dong J, Lele SM et al. YAP regulates cell proliferation, migration, and steroidogenesis in adult granulosa cell tumors. Endocr Relat Cancer 2014; 21: 297–310.
He C, Lv X, Hua G, Lele SM, Remmenga S, Dong J et al. YAP forms autocrine loops with the ERBB pathway to regulate ovarian cancer initiation and progression. Oncogene 2015; e-pub ahead of print 23 March 2015; doi:10.1038/onc.2015.52.
Jazaeri AA, Bryant JL, Park H, Li H, Dahiya N, Stoler MH et al. Molecular requirements for transformation of fallopian tube epithelial cells into serous carcinoma. Neoplasia 2011; 13: 899–911.
Karst AM, Levanon K, Drapkin R . Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proc Natl Acad Sci USA 2011; 108: 7547–7552.
Ando H, Kobayashi M, Toda S, Kikkawa F, Masahashi T, Mizutani S . Establishment of a ciliated epithelial cell line from human Fallopian tube. Hum Reprod 2000; 15: 1597–1603.
Smith JA, Madden T, Vijjeswarapu M, Newman RA . Inhibition of export of fibroblast growth factor-2 (FGF-2) from the prostate cancer cell lines PC3 and DU145 by Anvirzel and its cardiac glycoside component, oleandrin. Biochem Pharmacol 2001; 62: 469–472.
Guagnano V, Furet P, Spanka C, Bordas V, Le Douget M, Stamm C et al. Discovery of 3-(2,6-dichloro-3,5-dimethoxy-phenyl)-1-{6-[4-(4-ethyl-piperazin-1-yl)-phenylamin o]-pyrimidin-4-yl}-1-methyl-urea (NVP-BGJ398), a potent and selective inhibitor of the fibroblast growth factor receptor family of receptor tyrosine kinase. J Med Chem 2011; 54: 7066–7083.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013; 6: pl1.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012; 2: 401–404.
Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature 2012; 490: 61–70.
Cancer Genome Atlas Research N, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013; 497: 67–73.
Karst AM, Drapkin R . Primary culture and immortalization of human fallopian tube secretory epithelial cells. Nat Protoc 2012; 7: 1755–1764.
Perets R, Wyant GA, Muto KW, Bijron JG, Poole BB, Chin KT et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell 2013; 24: 751–765.
Kuhn E, Meeker A, Wang TL, Sehdev AS, Kurman RJ, Shih IeM . Shortened telomeres in serous tubal intraepithelial carcinoma: an early event in ovarian high-grade serous carcinogenesis. Am J Surg Pathol 2010; 34: 829–836.
Bowen NJ, Logani S, Dickerson EB, Kapa LB, Akhtar M, Benigno BB et al. Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol Oncol 2007; 104: 331–337.
Laury AR, Hornick JL, Perets R, Krane JF, Corson J, Drapkin R et al. PAX8 reliably distinguishes ovarian serous tumors from malignant mesothelioma. Am J Surg Pathol 2010; 34: 627–635.
Madore J, Ren F, Filali-Mouhim A, Sanchez L, Kobel M, Tonin PN et al. Characterization of the molecular differences between ovarian endometrioid carcinoma and ovarian serous carcinoma. J Pathol 2010; 220: 392–400.
Cathro HP, Stoler MH . Expression of cytokeratins 7 and 20 in ovarian neoplasia. Am J Clin Pathol 2002; 117: 944–951.
Tung CS, Mok SC, Tsang YT, Zu Z, Song H, Liu J et al. PAX2 expression in low malignant potential ovarian tumors and low-grade ovarian serous carcinomas. Mod Pathol 2009; 22: 1243–1250.
Crickard K, Gross JL, Crickard U, Yoonessi M, Lele S, Herblin WF et al. Basic fibroblast growth factor and receptor expression in human ovarian cancer. Gynecol Oncol 1994; 55: 277–284.
Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev 2012; 26: 1300–1305.
Zhang J, Ji JY, Yu M, Overholtzer M, Smolen GA, Wang R et al. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol 2009; 11: 1444–1450.
Yang S, Zhang L, Liu M, Chong R, Ding SJ, Chen Y et al. CDK1 phosphorylation of YAP promotes mitotic defects and cell motility and is essential for neoplastic transformation. Cancer Res 2013; 73: 6722–6733.
Fearon AE, Gould CR, Grose RP . FGFR signalling in women's cancers. Int J Biochem Cell Biol 2013; 45: 2832–2842.
Domcke S, Sinha R, Levine DA, Sander C, Schultz N . Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun 2013; 4: 2126.
Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA . Spheroid-based drug screen: considerations and practical approach. Nat Protoc 2009; 4: 309–324.
Stewart SL, Wike JM, Foster SL, Michaud F . The incidence of primary fallopian tube cancer in the United States. Gynecol Oncol 2007; 107: 392–397.
Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol 2007; 19: 3–9.
Dubeau L, Drapkin R . Coming into focus: the nonovarian origins of ovarian cancer. Ann Oncol 2013; 24: viii28–viii35.
Levanon K, Crum C, Drapkin R . New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol 2008; 26: 5284–5293.
Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007; 130: 1120–1133.
Lee KP, Lee JH, Kim TS, Kim TH, Park HD, Byun JS et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci USA 2010; 107: 8248–8253.
Harvey KF, Zhang X, Thomas DM . The Hippo pathway and human cancer. Nat Rev Cancer 2013; 13: 246–257.
Cai H, Xu Y . The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells. Cell Commun Signal 2013; 11: 31.
Yuan M, Tomlinson V, Lara R, Holliday D, Chelala C, Harada T et al. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ 2008; 15: 1752–1759.
Strano S, Monti O, Pediconi N, Baccarini A, Fontemaggi G, Lapi E et al. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA Damage. Mol Cell 2005; 18: 447–459.
Kendall SD, Linardic CM, Adam SJ, Counter CM . A network of genetic events sufficient to convert normal human cells to a tumorigenic state. Cancer Res 2005; 65: 9824–9828.
Shan W, Mercado-Uribe I, Zhang J, Rosen D, Zhang S, Wei J et al. Mucinous adenocarcinoma developed from human fallopian tube epithelial cells through defined genetic modifications. Cell Cycle 2012; 11: 2107–2113.
Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature 2011; 474: 609–615.
Hwang H, Quenneville L, Yaziji H, Gown AM . Wilms tumor gene product: sensitive and contextually specific marker of serous carcinomas of ovarian surface epithelial origin. Appl Immunohistochem Mol Morphol 2004; 12: 122–126.
Al-Hussaini M, Stockman A, Foster H, McCluggage WG . WT-1 assists in distinguishing ovarian from uterine serous carcinoma and in distinguishing between serous and endometrioid ovarian carcinoma. Histopathology 2004; 44: 109–115.
Kim J, Coffey DM, Creighton CJ, Yu Z, Hawkins SM, Matzuk MM . High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci USA 2012; 109: 3921–3926.
Sherman-Baust CA, Kuhn E, Valle BL, Shih IeM, Kurman RJ, Wang TL et al. A genetically engineered ovarian cancer mouse model based on fallopian tube transformation mimics human high-grade serous carcinoma development. J Pathol 2014; 233: 228–237.
Dailey L, Ambrosetti D, Mansukhani A, Basilico C . Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev 2005; 16: 233–247.
Eswarakumar VP, Lax I, Schlessinger J . Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev 2005; 16: 139–149.
De Cecco L, Marchionni L, Gariboldi M, Reid JF, Lagonigro MS, Caramuta S et al. Gene expression profiling of advanced ovarian cancer: characterization of a molecular signature involving fibroblast growth factor 2. Oncogene 2004; 23: 8171–8183.
Di Blasio AM, Cremonesi L, Vigano P, Ferrari M, Gospodarowicz D, Vignali M et al. Basic fibroblast growth factor and its receptor messenger ribonucleic acids are expressed in human ovarian epithelial neoplasms. Am J Obstet Gynecol 1993; 169: 1517–1523.
Li T, Jiang S . Effect of bFGF on invasion of ovarian cancer cells through the regulation of Ets-1 and urokinase-type plasminogen activator. Pharm Biol 2010; 48: 161–165.
Meyer GE, Yu E, Siegal JA, Petteway JC, Blumenstein BA, Brawer MK . Serum basic fibroblast growth factor in men with and without prostate carcinoma. Cancer 1995; 76: 2304–2311.
Ono I . The effects of basic fibroblast growth factor (bFGF) on the breaking strength of acute incisional wounds. J Dermatol Sci 2002; 29: 104–113.
Beenken A, Mohammadi M . The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov 2009; 8: 235–253.
Wang C, Roy SK . Expression of E-cadherin and N-cadherin in perinatal hamster ovary: possible involvement in primordial follicle formation and regulation by follicle-stimulating hormone. Endocrinology 2010; 151: 2319–2330.
Wang C, Lv X, He C, Hua G, Tsai MY, Davis JS . The G-protein-coupled estrogen receptor agonist G-1 suppresses proliferation of ovarian cancer cells by blocking tubulin polymerization. Cell Death Dis 2013; 4: e869.
Wang C, Lv X, Jiang C, Cordes CM, Fu L, Lele SM et al. Transforming growth factor alpha (TGFalpha) regulates granulosa cell tumor (GCT) cell proliferation and migration through activation of multiple pathways. PLoS One 2012; 7: e48299.
Siffroi-Fernandez S, Cinaroglu A, Fuhrmann-Panfalone V, Normand G, Bugra K, Sahel J et al. Acidic fibroblast growth factor (FGF-1) and FGF receptor 1 signaling in human Y79 retinoblastoma. Arch Ophthalmol 2005; 123: 368–376.
Krejci P, Krakow D, Mekikian PB, Wilcox WR . Fibroblast growth factors 1, 2, 17, and 19 are the predominant FGF ligands expressed in human fetal growth plate cartilage. Pediatr Res 2007; 61: 267–272.
Beaufort CM, Helmijr JC, Piskorz AM, Hoogstraat M, Ruigrok-Ritstier K, Besselink N et al. Ovarian cancer cell line panel (OCCP): clinical importance of in vitro morphological subtypes. PLoS One 2014; 9: e103988.
Acknowledgements
We thank Dr Adam Karpf (The Pamela and Fred Cancer Center, University of Nebraska Medical Center) for providing ovarian HGSC cell lines. We acknowledge the cBioPortal for Cancer Genomics (http://cbioportal.org) for providing data sets and online analyzing tools. We also acknowledge the TCGA research Network (http://cancergenome.nih.gov) for providing data sets. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (5R00HD059985, 5P01AG029531); the Olson Center for Women’s Health (no number); the Fred and Pamela Buffett Cancer Center (LB595), the Colleen’s Dream Foundation (no number), the Omaha Veterans Administration Medical Center (5I01BX000512) and the National Institute of Food and Agriculture (2011-67015-20076). We thank Melody Montgomery at the University of Nebraska Medical Center (UNMC) Research Editorial Office for the professional editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Hua, G., Lv, X., He, C. et al. YAP induces high-grade serous carcinoma in fallopian tube secretory epithelial cells. Oncogene 35, 2247–2265 (2016). https://doi.org/10.1038/onc.2015.288
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.288
This article is cited by
-
KIAA1429-mediated m6A modification of CHST11 promotes progression of diffuse large B-cell lymphoma by regulating Hippo–YAP pathway
Cellular & Molecular Biology Letters (2023)
-
Increasing cancer permeability by photodynamic priming: from microenvironment to mechanotransduction signaling
Cancer and Metastasis Reviews (2022)
-
Radiation-induced YAP activation confers glioma radioresistance via promoting FGF2 transcription and DNA damage repair
Oncogene (2021)
-
Synergistic enhancement of efficacy of platinum drugs with verteporfin in ovarian cancer cells
BMC Cancer (2020)
-
A dog oviduct-on-a-chip model of serous tubal intraepithelial carcinoma
Scientific Reports (2020)