Abstract
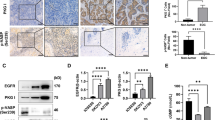
p21-activated kinases (Paks) are Cdc42/Rac-activated serine–threonine protein kinases that regulate several key cancer-relevant signaling pathways, such as the Mek/Erk, PI3K/Akt and Wnt/b-catenin signaling pathways. Pak1 is frequently overexpressed and/or hyperactivated in different human cancers, including human breast, ovary, prostate and brain cancer, due to amplification of the PAK1 gene in an 11q13 amplicon. Genetic or pharmacological inactivation of Pak1 has been shown to reduce proliferation of different cancer cells in vitro and reduce tumor progression in vivo. In this work, we examined the roles of Pak1 in cellular and animal models of PAK1-amplified ovarian cancer. We found that inhibition of Pak1 leads to decreased proliferation and migration in PAK1-amplified/overexpressed ovarian cancer cells, and has no effect in cell that lack such amplification/overexpression. Further, we observed that loss of Pak1 function causes 11q13-amplified ovarian cancer cells to arrest in the G2/M phase of the cell cycle. This arrest correlates with activation of p53 and p21Cip and decreased expression of cyclin B1. These findings suggest that small-molecule inhibitors of Pak1 may have a therapeutic role in the ~25% of ovarian cancers characterized by PAK1 gene amplification.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Radu M, Semenova G, Kosoff R, Chernoff J . PAK signalling during the development and progression of cancer. Nat Rev Cancer 2014; 14: 13–25.
Arias-Romero LE, Chernoff J . A tale of two Paks. Biol Cell 2008; 100: 97–108.
Prudnikova TY, Rawat SJ, Chernoff J . Molecular pathways: targeting the kinase effectors of RHO-family GTPases. Clin Cancer Res 2015; 21: 24–29.
Wang Z, Fu M, Wang L, Liu J, Li Y, Brakebusch C et al. p21-activated kinase 1 (PAK1) can promote ERK activation in a kinase-independent manner. J Biol Chem 2013; 288: 20093–20099.
Wang Z, Pedersen E, Basse A, Lefever T, Peyrollier K, Kapoor S et al. Rac1 is crucial for Ras-dependent skin tumor formation by controlling Pak1-Mek-Erk hyperactivation and hyperproliferation in vivo. Oncogene 2010; 29: 3362–3373.
Arias-Romero LE, Villamar-Cruz O, Huang M, Hoeflich KP, Chernoff J . Pak1 kinase links ErbB2 to beta-catenin in transformation of breast epithelial cells. Cancer Res 2013; 73: 3671–3682.
He H, Shulkes A, Baldwin GS . PAK1 interacts with beta-catenin and is required for the regulation of the beta-catenin signalling pathway by gastrins. Biochim Biophys Acta 2008; 1783: 1943–1954.
Higuchi M, Onishi K, Kikuchi C, Gotoh Y . Scaffolding function of PAK in the PDK1-Akt pathway. Nat Cell Biol 2008; 10: 1356–1364.
Schurmann A, Mooney AF, Sanders LC, Sells MA, Wang HG, Reed JC et al. p21-activated kinase 1 phosphorylates the death agonist bad and protects cells from apoptosis. Mol Cell Biol 2000; 20: 453–461.
Tran NH, Frost JA . Phosphorylation of Raf-1 by p21-activated kinase 1 and Src regulates Raf-1 autoinhibition. J Biol Chem 2003; 278: 11221–11226.
Motwani M, Li D-Q, Horvath A, Kumar R . Identification of novel gene targets and functions of p21-activated kinase 1 during DNA damage by gene expression profiling. PLoS One 2013; 8: e66585.
Shrestha Y, Schafer EJ, Boehm JS, Thomas SR, He F, Du J et al. PAK1 is a breast cancer oncogene that coordinately activates MAPK and MET signaling. Oncogene 2012; 31: 3397–3408.
Brown LA, Kalloger SE, Miller MA, Shih Ie M, McKinney SE, Santos JL et al. Amplification of 11q13 in ovarian carcinoma. Genes Chromosomes Cancer 2008; 47: 481–489.
Lundgren K, Holm K, Nordenskjold B, Borg A, Landberg G . Gene products of chromosome 11q and their association with CCND1 gene amplification and tamoxifen resistance in premenopausal breast cancer. Breast Cancer Res 2008; 10: R81.
Arias-Romero LE, Villamar-Cruz O, Pacheco A, Kosoff R, Huang M, Muthuswamy SK et al. A Rac-Pak signaling pathway is essential for ErbB2-mediated transformation of human breast epithelial cancer cells. Oncogene 2010; 29: 5839–5849.
Dadke D, Fryer BH, Golemis EA, Field J . Activation of p21-activated kinase 1-nuclear factor kappaB signaling by Kaposi's sarcoma-associated herpes virus G protein-coupled receptor during cellular transformation. Cancer Res 2003; 63: 8837–8847.
Tang Y, Chen Z, Ambrose D, Liu J, Gibbs JB, Chernoff J et al. Kinase-deficient Pak1 mutants inhibit Ras transformation of Rat-1 fibroblasts. Mol Cell Biol 1997; 17: 4454–4464.
Ong CC, Jubb AM, Haverty PM, Zhou W, Tran V, Truong T et al. Targeting p21-activated kinase 1 (PAK1) to induce apoptosis of tumor cells. Proc Natl Acad Sci USA 2011; 108: 7177–7182.
Lambros MB, Fiegler H, Jones A, Gorman P, Roylance RR, Carter NP et al. Analysis of ovarian cancer cell lines using array-based comparative genomic hybridization. J Pathol 2005; 205: 29–40.
Abbas T, Dutta A . p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 2009; 9: 400–414.
de Feraudy S, Revet I, Bezrookove V, Feeney L, Cleaver JE . A minority of foci or pan-nuclear apoptotic staining of gammaH2AX in the S phase after UV damage contain DNA double-strand breaks. Proc Natl Acad Sci USA 2010; 107: 6870–6875.
Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A . H2AX: the histone guardian of the genome. DNA Repair 2004; 3: 959–967.
Fan S, Yuan R, Ma YX, Xiong J, Meng Q, Erdos M et al. Disruption of BRCA1 LXCXE motif alters BRCA1 functional activity and regulation of RB family but not RB protein binding. Oncogene 2001; 20: 4827–4841.
Yi C, Wilker EW, Yaffe MB, Stemmer-Rachamimov A, Kissil JL . Validation of the p21-activated kinases as targets for inhibition in neurofibromatosis type 2. Cancer Res 2008; 68: 7932–7937.
Murray BW, Guo C, Piraino J, Westwick JK, Zhang C, Lamerdin J et al. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc Natl Acad Sci USA 2010; 107: 9446–9451.
Chow HY, Dong B, Duron SG, Campbell DA, Ong CC, Hoeflich KP et al. Group I Paks as therapeutic targets in NF2-deficient meningioma. Oncotarget 2014; 6: 1981–1994.
Choi JH, Sheu JJ, Guan B, Jinawath N, Markowski P, Wang TL et al. Functional analysis of 11q13.5 amplicon identifies Rsf-1 (HBXAP) as a gene involved in paclitaxel resistance in ovarian cancer. Cancer Res 2009; 69: 1407–1415.
Davidson B, Shih Ie M, Wang TL . Different clinical roles for p21-activated kinase-1 in primary and recurrent ovarian carcinoma. Hum Pathol 2008; 39: 1630–1636.
Holm K, Staaf J, Jonsson G, Vallon-Christersson J, Gunnarsson H, Arason A et al. Characterisation of amplification patterns and target genes at chromosome 11q13 in CCND1-amplified sporadic and familial breast tumours. Breast Cancer Res Treat 2012; 133: 583–594.
Huynh N, Liu KH, Baldwin GS, He H . P21-activated kinase 1 stimulates colon cancer cell growth and migration/invasion via ERK- and AKT-dependent pathways. Biochim Biophys Acta 2010; 1803: 1106–1113.
He H, Huynh N, Liu KH, Malcontenti-Wilson C, Zhu J, Christophi C et al. P-21 activated kinase 1 knockdown inhibits beta-catenin signalling and blocks colorectal cancer growth. Cancer Lett 2012; 317: 65–71.
Liu S, Goldstein RH, Scepansky EM, Rosenblatt M . Inhibition of rho-associated kinase signaling prevents breast cancer metastasis to human bone. Cancer Res 2009; 69: 8742–8751.
Park ER, Eblen ST, Catling AD . MEK1 activation by PAK: A novel mechanism. Cell Signal 2007; 19: 1488–1496.
Bullock AN, Henckel J, DeDecker BS, Johnson CM, Nikolova PV, Proctor MR et al. Thermodynamic stability of wild-type and mutant p53 core domain. Proc Natl Acad Sci USA 1997; 94: 14338–14342.
Merabet A, Houlleberghs H, Maclagan K, Akanho E, Bui TT, Pagano B et al. Mutants of the tumour suppressor p53 L1 loop as second-site suppressors for restoring DNA binding to oncogenic p53 mutations: structural and biochemical insights. Biochem J 2010; 427: 225–236.
Wu J, Li QQ, Zhou H, Lu Y, Li JM, Ma Y et al. Selective tumor cell killing by triptolide in p53 wild-type and p53 mutant ovarian carcinomas. Med Oncol 2014; 31: 14.
Guan YQ, Li Z, Yang A, Huang Z, Zheng Z, Zhang L et al. Cell cycle arrest and apoptosis of OVCAR-3 and MCF-7 cells induced by co-immobilized TNF-alpha plus IFN-gamma on polystyrene and the role of p53 activation. Biomaterials 2012; 33: 6162–6171.
Yarden RI, Brody LC . BRCA1 interacts with components of the histone deacetylase complex. Proc Natl Acad Sci USA 1999; 96: 4983–4988.
Aprelikova ON, Fang BS, Meissner EG, Cotter S, Campbell M, Kuthiala A et al. BRCA1-associated growth arrest is RB-dependent. Proc Natl Acad Sci USA 1999; 96: 11866–11871.
Chow HY, Jubb AM, Koch JN, Jaffer ZM, Stepanova D, Campbell DA et al. p21-Activated kinase 1 is required for efficient tumor formation and progression in a Ras-mediated skin cancer model. Cancer Res 2012; 72: 5966–5975.
Hashimoto H, Sudo T, Maruta H, Nishimura R . The direct PAK1 inhibitor, TAT-PAK18, blocks preferentially the growth of human ovarian cancer cell lines in which PAK1 is abnormally activated by autophosphorylation at Thr 423. Drug Discov Ther 2010; 4: 1–4.
Hashimoto H, Messerli SM, Sudo T, Maruta H . Ivermectin inactivates the kinase PAK1 and blocks the PAK1-dependent growth of human ovarian cancer and NF2 tumor cell lines. Drug Discov Ther 2009; 3: 243–246.
Limame R, Wouters A, Pauwels B, Fransen E, Peeters M, Lardon F et al. Comparative analysis of dynamic cell viability, migration and invasion assessments by novel real-time technology and classic endpoint assays. PLoS One 2012; 7: e46536.
Acknowledgements
We thank M. Einarson (Fox Chase Cancer Center, High Throughput and Translational Core Facility) for assistance with cell cycle analysis and the Cell Culture Facility. We thank D. Connolly (FCCC), N. Tonks (CSHL) and G. Mills (MD Anderson) for ovarian cancer cell lines and for insightful comments on the manuscript. This work was supported by grants from the National Institutes of Health to JC (R01-CA142928 and R01-CA148805), Ovarian SPORE P50 CA083638 (Pilot Award to JC), Fox Chase Cancer Center (P30-CA006927), as well as by an appropriation from the State of Pennsylvania.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Prudnikova, T., Villamar-Cruz, O., Rawat, S. et al. Effects of p21-activated kinase 1 inhibition on 11q13-amplified ovarian cancer cells. Oncogene 35, 2178–2185 (2016). https://doi.org/10.1038/onc.2015.278
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.278
This article is cited by
-
HACE1-mediated NRF2 activation causes enhanced malignant phenotypes and decreased radiosensitivity of glioma cells
Signal Transduction and Targeted Therapy (2021)
-
Targeting p21-activated kinase 1 inhibits growth and metastasis via Raf1/MEK1/ERK signaling in esophageal squamous cell carcinoma cells
Cell Communication and Signaling (2019)
-
Targeting PKCι-PAK1 signaling pathways in EGFR and KRAS mutant adenocarcinoma and lung squamous cell carcinoma
Cell Communication and Signaling (2019)
-
Trabectedin triggers direct and NK-mediated cytotoxicity in multiple myeloma
Journal of Hematology & Oncology (2019)
-
Combined inhibition of Aurora A and p21-activated kinase 1 as a new treatment strategy in breast cancer
Breast Cancer Research and Treatment (2019)