Abstract
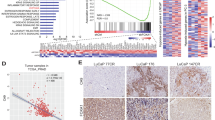
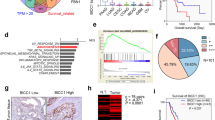
Metastasis of tumors requires angiogenesis, which is comprised of multiple biological processes that are regulated by angiogenic factors. The fibroblast growth factor (FGF) is a potent angiogenic factor and aberrant FGF signaling is a common property of tumors. Yet, how the aberration in cancer cells contributes to angiogenesis in the tumor is not well understood. Most studies of its angiogenic signaling mechanisms have been in endothelial cells. FGF receptor substrate 2α (FRS2α) is an FGF receptor-associated protein required for activation of downstream signaling molecules that include those in the mitogen-activated protein and AKT kinase pathways. Herein, we demonstrated that overactivation and hyperactivity of FRS2α, as well as overexpression of cJUN and HIF1α, were positively correlated with vessel density and progression of human prostate cancer (PCa) toward malignancy. We also demonstrate that FGF upregulated the production of vascular endothelial growth factor A mainly by increasing expression of cJUN and HIF1α. This then promoted recruitment of endothelial cells and vessel formation for the tumor. Tumor angiogenesis in mouse PCa tissues was compromised by tissue-specific ablation of Frs2α in prostate epithelial cells. Depletion of Frs2α expression in human PCa cells and in a preclinical xenograft model, MDA PCa 118b, also significantly suppressed tumor angiogenesis accompanied with decreased tumor growth in the bone. The results underscore the angiogenic role of FRS2α-mediated signaling in tumor epithelial cells in angiogenesis. They provide a rationale for treating PCa with inhibitors of FGF signaling. They also demonstrate the potential of overexpressed FRS2α as a biomarker for PCa diagnosis, prognosis and response to therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
McDougall SR, Anderson AR, Chaplain MA . Mathematical modelling of dynamic adaptive tumour-induced angiogenesis: clinical implications and therapeutic targeting strategies. J Theor Biol 2006; 241: 564–589.
Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J . Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol 1993; 143: 401–409.
Harris AL, Zhang H, Moghaddam A, Fox S, Scott P, Pattison A et al. Breast cancer angiogenesis—new approaches to therapy via antiangiogenesis, hypoxic activated drugs, and vascular targeting. Breast Cancer Res Treat 1996; 38: 97–108.
Yu EM, Jain M, Aragon-Ching JB . Angiogenesis inhibitors in prostate cancer therapy. Discov Med 2010; 10: 521–530.
Cross MJ, Claesson-Welsh L . FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci 2001; 22: 201–207.
McKeehan WL, Wang F, Luo Y The fibroblast growth factor (FGF) signaling complex Handbook of Cell Signaling 2dn edn vol. I. Academic/Elsevier Press: New York, NY, USA, 2009.
Gotoh N, Ito M, Yamamoto S, Yoshino I, Song N, Wang Y et al. Tyrosine phosphorylation sites on FRS2alpha responsible for Shp2 recruitment are critical for induction of lens and retina. Proc Natl Acad Sci USA 2004; 101: 17144–17149.
Hadari YR, Gotoh N, Kouhara H, Lax I, Schlessinger J . Critical role for the docking-protein FRS2 alpha in FGF receptor-mediated signal transduction pathways. Proc Natl Acad Sci USA 2001; 98: 8578–8583.
Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010; 18: 11–22.
Devilard E, Bladou F, Ramuz O, Karsenty G, Dales JP, Gravis G et al. FGFR1 and WT1 are markers of human prostate cancer progression. BMC Cancer 2006; 6: 272.
Giri D, Ropiquet F, Ittmann M . Alterations in expression of basic fibroblast growth factor (FGF) 2 and its receptor FGFR-1 in human prostate cancer. Clin Cancer Res 1999; 5: 1063–1071.
Ozen M, Giri D, Ropiquet F, Mansukhani A, Ittmann M . Role of fibroblast growth factor receptor signaling in prostate cancer cell survival. J Natl Cancer Inst 2001; 93: 1783–1790.
Wang J, Stockton DW, Ittmann M . The fibroblast growth factor receptor-4 Arg388 allele is associated with prostate cancer initiation and progression. Clin Cancer Res 2004; 10: 6169–6178.
Acevedo VD, Gangula RD, Freeman KW, Li R, Zhang Y, Wang F et al. Inducible FGFR-1 activation leads to irreversible prostate adenocarcinoma and an epithelial-to-mesenchymal transition. Cancer Cell 2007; 12: 559–571.
Giri D, Ropiquet F, Ittmann M . FGF9 is an autocrine and paracrine prostatic growth factor expressed by prostatic stromal cells. J Cell Physiol 1999; 180: 53–60.
Jin C, McKeehan K, Guo W, Jauma S, Ittmann MM, Foster B et al. Cooperation between ectopic FGFR1 and depression of FGFR2 in induction of prostatic intraepithelial neoplasia in the mouse prostate. Cancer Res 2003; 63: 8784–8790.
Li ZG, Mathew P, Yang J, Starbuck MW, Zurita AJ, Liu J et al. Androgen receptor-negative human prostate cancer cells induce osteogenesis in mice through FGF9-mediated mechanisms. J Clin Invest 2008; 118: 2697–2710.
Memarzadeh S, Xin L, Mulholland DJ, Mansukhani A, Wu H, Teitell MA et al. Enhanced paracrine FGF10 expression promotes formation of multifocal prostate adenocarcinoma and an increase in epithelial androgen receptor. Cancer Cell 2007; 12: 572–585.
Polnaszek N, Kwabi-Addo B, Peterson LE, Ozen M, Greenberg NM, Ortega S et al. Fibroblast growth factor 2 promotes tumor progression in an autochthonous mouse model of prostate cancer. Cancer Res 2003; 63: 5754–5760.
Song Z, Powell WC, Kasahara N, van Bokhoven A, Miller GJ, Roy-Burman P . The effect of fibroblast growth factor 8, isoform b, on the biology of prostate carcinoma cells and their interaction with stromal cells. Cancer Res 2000; 60: 6730–6736.
Valta MP, Tuomela J, Bjartell A, Valve E, Vaananen HK, Harkonen P . FGF-8 is involved in bone metastasis of prostate cancer. Int J Cancer 2008; 123: 22–31.
Wang F, McKeehan K, Yu C, Ittmann M, McKeehan WL . Chronic activity of ectopic type 1 fibroblast growth factor receptor tyrosine kinase in prostate epithelium results in hyperplasia accompanied by intraepithelial neoplasia. Prostate 2004; 58: 1–12.
Yang F, Zhang Y, Ressler SJ, Ittmann MM, Ayala GE, Dang TD et al. FGFR1 is essential for prostate cancer progression and metastasis. Cancer Res 2013; 73: 3716–3724.
Zhang Y, Zhang J, Lin Y, Lan Y, Lin C, Xuan JW et al. Role of epithelial cell fibroblast growth factor receptor substrate 2{alpha} in prostate development, regeneration and tumorigenesis. Development 2008; 135: 775–784.
Winter SF, Acevedo VD, Gangula RD, Freeman KW, Spencer DM, Greenberg NM . Conditional activation of FGFR1 in the prostate epithelium induces angiogenesis with concomitant differential regulation of Ang-1 and Ang-2. Oncogene 2007; 26: 4897–4907.
Teishima J, Yano S, Shoji K, Hayashi T, Goto K, Kitano H et al. Accumulation of FGF9 in prostate cancer correlates with epithelial-to-mesenchymal transition and induction of VEGF-A expression. Anticancer Res 2014; 34: 695–700.
Bergers G, Benjamin LE . Angiogenesis: Tumorigenesis and the angiogenic switch. Nat Rev Cancer 2003; 3: 401–410.
Francescone RA III, Faibish M, Shao R . A Matrigel-based tube formation assay to assess the vasculogenic activity of tumor cells. J Vis Exp 2011; 7.
Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN . Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 1998; 93: 705–716.
Wang X, Asmann YW, Erickson-Johnson MR, Oliveira JL, Zhang H, Moura RD et al. High-resolution genomic mapping reveals consistent amplification of the fibroblast growth factor receptor substrate 2 gene in well-differentiated and dedifferentiated liposarcoma. Genes chromosomes Cancer 2011; 50: 849–858.
Zhang K, Chu K, Wu X, Gao H, Wang J, Yuan YC et al. Amplification of FRS2 and activation of FGFR/FRS2 signaling pathway in high-grade liposarcoma. Cancer Res 2013; 73: 1298–1307.
Valencia T, Joseph A, Kachroo N, Darby S, Meakin S, Gnanapragasam VJ . Role and expression of FRS2 and FRS3 in prostate cancer. BMC Cancer 2011; 11: 484.
Huang X, Yu C, Jin C, Kobayashi M, Bowles CA, Wang F et al. Ectopic activity of fibroblast growth factor receptor 1 in hepatocytes accelerates hepatocarcinogenesis by driving proliferation and vascular endothelial growth factor-induced angiogenesis. Cancer Res 2006; 66: 1481–1490.
Wan X, Corn PG, Yang J, Palanisamy N, Starbuck MW, Efstathiou E et al. Prostate cancer cell-stromal cell crosstalk via FGFR1 mediates antitumor activity of dovitinib in bone metastases. Science Transl Med 2014; 6 252ra122.
Pages G, Pouyssegur J . Transcriptional regulation of the Vascular Endothelial Growth Factor gene—a concert of activating factors. Cardiovasc Res 2005; 65: 564–573.
Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem 1991; 266: 11947–11954.
Jin C, McKeehan K, Wang F . Transgenic mouse with high Cre recombinase activity in all prostate lobes, seminal vesicle, and ductus deferens. Prostate 2003; 57: 160–164.
Lin Y, Zhang J, Zhang Y, Wang F . Generation of an Frs2alpha conditional null allele. Genesis 2007; 45: 554–559.
Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA 1995; 92: 3439–3443.
Zhong WD, Liang YX, Lin SX, Li L, He HC, Bi XC et al. Expression of CD147 is associated with prostate cancer progression. Int J Cancer 2012; 130: 300–308.
Huang YQ, Han ZD, Liang YX, Lin ZY, Ling XH, Fu X et al. Decreased expression of myosin light chain MYL9 in stroma predicts malignant progression and poor biochemical recurrence-free survival in prostate cancer. Med Oncol 2014; 31: 820.
Acknowledgements
We thank Dr Stefan Siwko and Samantha Del Castillo for critical reading of the manuscript. This work was supported in part by the National Institutes of Health CA96824 and DE023106 to FW, CA140388 to NN, WLM and FW, and The Cancer Prevention and Research Institution of Texas CPRIT110555 to FW and WLM, and the National Natural Science Foundation of China 81170699, 81272813 to CW, 81101712, 81270761 to XL, 510180 to WZ and 81072016 to ZYZ.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Liu, J., You, P., Chen, G. et al. Hyperactivated FRS2α-mediated signaling in prostate cancer cells promotes tumor angiogenesis and predicts poor clinical outcome of patients. Oncogene 35, 1750–1759 (2016). https://doi.org/10.1038/onc.2015.239
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.239
This article is cited by
-
Discovery of a small molecule ligand of FRS2 that inhibits invasion and tumor growth
Cellular Oncology (2023)
-
A novel risk score model based on five angiogenesis-related long non-coding RNAs for bladder urothelial carcinoma
Cancer Cell International (2022)
-
Influence of SHH/GLI1 axis on EMT mediated migration and invasion of breast cancer cells
Scientific Reports (2019)