Abstract
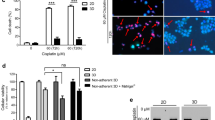
Preclinical studies of anticancer drugs are typically performed using cancer cell lines maintained in two-dimensional (2D) cultures, ignoring the influences of the extracellular matrix (ECM) and three-dimensional (3D) microenvironment. In this study, we evaluated the microenvironmental control of human breast cancer cells responses to doxorubicin (DOXO) using the 3D laminin-rich ECM (3D lrECM) cell culture model. Under 3D culture conditions, MCF-7 cells displayed drastic morphological alterations, a decrease in proliferation and elevated sensitivity to DOXO. Interestingly, the chemotherapy-mediated activation of autophagy was compromised in the 3D matrix, suggesting an association between the increased cytotoxicity of DOXO and hindered autophagy induction. Indeed, while chloroquine or ATG5 knockdown potentiated DOXO-induced cell death under the 2D culture conditions, the autophagy inducer rapamycin improved the resistance of 3D-cultured cells to this drug. Moreover, in the monolayer-cultured cells, DOXO treatment led to increases in p53 and DRAM-1 expression, which is a p53-dependent activator of autophagy that functions in response to DNA damage. Conversely, p53 and DRAM-1 expression was impaired in 3D-cultured cells. The knockdown of p53 by shRNA blocked DRAM-1 activation, impaired autophagy induction and sensitized only those cells maintained under 2D conditions to DOXO. In addition, 2D-cultured MDA-MB-231 cells (a p53-mutated breast cancer cell line) not only showed increased sensitivity to DOXO compared with MCF-7 cells but also failed to induce DRAM-1 expression or autophagy. Similar to p53 silencing, DRAM-1 knockdown potentiated DOXO cytotoxicity only in 2D-cultured cells. These results suggest that the 3D tissue microenvironment controls tumor cell sensitivity to DOXO treatment by preventing p53-DRAM-autophagy axis activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A . Cancer statistics 2012 CA Cancer J Clin 2012; 62: 10–29.
Khasraw M, Bell R, Dang C . Epirubicin: is it like doxorubicin in breast cancer? A clinical review. Breast 2012; 21: 142–149.
DeSantis C, Ma J, Bryan L, Jemal A . Breast cancer statistics, 2013. CA Cancer J Clin 2014; 64: 52–62.
Ahn JH, Lee M . Suppression of autophagy sensitizes multidrug resistant cells towards Src tyrosine kinase specific inhibitor PP2. Cancer Lett 2011; 310: 188–197.
Liang B, Kong D, Liu Y, Liang N, He M, Ma S et al. Autophagy inhibition plays the synergetic killing roles with radiation in the multi-drug resistant SKVCR ovarian cancer cells. Radiat Oncol 2012; 7: 213.
Levine B, Kroemer G . Autophagy in the pathogenesis of disease. Cell 2008; 132: 27–42.
Singh R, Cuervo AM . Autophagy in the cellular energetic balance. Cell Metab 2011; 13: 495–504.
Chen Y, Azad MB, Gibson SB . Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ 2009; 16: 1040–1052.
Crighton D, Wilkinson S, O'Prey J, Syed N, Smith P, Harrison PR et al. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell 2006; 126: 121–134.
Manov I, Pollak Y, Broneshter R, Iancu TC . Inhibition of doxorubicin-induced autophagy in hepatocellular carcinoma Hep3B cells by sorafenib—the role of extracellular signal-regulated kinase counteraction. FEBS J 2011; 278: 3494–3507.
Mathew R, White E . Autophagy, stress, and cancer metabolism: what doesn't kill you makes you stronger. Cold Spring Harb Symp Quant Biol 2011; 76: 389–396.
Kimmelman AC . The dynamic nature of autophagy in cancer. Genes Dev 2011; 25: 1999–2010.
Qu X, Zou Z, Sun Q, Luby-Phelps K, Cheng P, Hogan RN et al. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell 2007; 128: 931–946.
Weaver VM, Bissell MJ . Functional culture models to study mechanisms governing apoptosis in normal and malignant mammary epithelial cells. J Mammary Gland Biol Neoplasia 1999; 4: 193–201.
Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, Spellman PT et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol 2007; 1: 84–96.
Lee GY, Kenny PA, Lee EH, Bissell MJ . Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods 2007; 4: 359–365.
Valbuena A, Castro-Obregon S, Lazo PA . Downregulation of VRK1 by p53 in response to DNA damage is mediated by the autophagic pathway. PLoS One 2011; 6: e17320.
Mohan P, Rapoport N . Doxorubicin as a molecular nanotheranostic agent: effect of doxorubicin encapsulation in micelles or nanoemulsions on the ultrasound-mediated intracellular delivery and nuclear trafficking. Mol Pharm 2010; 7: 1959–1973.
Li Q, Chow AB, Mattingly RR . Three-dimensional overlay culture models of human breast cancer reveal a critical sensitivity to mitogen-activated protein kinase kinase inhibitors. J Pharmacol Exp Ther 2010; 332: 821–828.
Woodley-Cook J, Shin LY, Swystun L, Caruso S, Beaudin S, Liaw PC . Effects of the chemotherapeutic agent doxorubicin on the protein C anticoagulant pathway. Mol Cancer Ther 2006; 5: 3303–3311.
Swenson CE, Bolcsak LE, Batist G, Guthrie TH Jr., Tkaczuk KH, Boxenbaum H et al. Pharmacokinetics of doxorubicin administered i.v. as Myocet (TLC D-99; liposome-encapsulated doxorubicin citrate) compared with conventional doxorubicin when given in combination with cyclophosphamide in patients with metastatic breast cancer. Anticancer Drugs 2003; 14: 239–246.
Bramwell VH, Morris D, Ernst DS, Hings I, Blackstein M, Venner PM et al. Safety and efficacy of the multidrug-resistance inhibitor biricodar (VX-710) with concurrent doxorubicin in patients with anthracycline-resistant advanced soft tissue sarcoma. Clin Cancer Res 2002; 8: 383–393.
Sun WL, Chen J, Wang YP, Zheng H . Autophagy protects breast cancer cells from epirubicin-induced apoptosis and facilitates epirubicin-resistance development. Autophagy 2011; 7: 1035–1044.
Mizushima N, Yoshimori T . How to interpret LC3 immunoblotting. Autophagy 2007; 3: 542–545.
Cortat B, Garcia CC, Quinet A, Schuch AP, de Lima-Bessa KM, Menck CF . The relative roles of DNA damage induced by UVA irradiation in human cells. Photochem Photobiol Sci 2013; 12: 1483–1495.
Moraes MC, de Andrade AQ, Carvalho H, Guecheva T, Agnoletto MH, Henriques JA et al. Both XPA and DNA polymerase eta are necessary for the repair of doxorubicin-induced DNA lesions. Cancer Lett 2012; 314: 108–118.
Cheng YJ, Lee CH, Lin YP, Huang JY, Su CC, Chang WT et al. Caspase-3 enhances lung metastasis and cell migration in a protease-independent mechanism through the ERK pathway. Int J Cancer 2008; 123: 1278–1285.
Pyo JO, Yoo SM, Ahn HH, Nah J, Hong SH, Kam TI et al. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat Commun 2013; 4: 2300.
Batista LF, Roos WP, Christmann M, Menck CF, Kaina B . Differential sensitivity of malignant glioma cells to methylating and chloroethylating anticancer drugs: p53 determines the switch by regulating xpc, ddb2, and DNA double-strand breaks. Cancer Res 2007; 67: 11886–11895.
Beckta JM, Ahmad SF, Yang H, Valerie K . Revisiting p53 for cancer-specific chemo- and radiotherapy: Ten years after. Cell Cycle 2014; 13: 710–713.
Bertheau P, Lehmann-Che J, Varna M, Dumay A, Poirot B, Porcher R et al. p53 in breast cancer subtypes and new insights into response to chemotherapy. Breast 2013; 22: S27–S29.
Li DD, Sun T, Wu XQ, Chen SP, Deng R, Jiang S et al. The inhibition of autophagy sensitises colon cancer cells with wild-type p53 but not mutant p53 to topotecan treatment. PLoS One 2012; 7: e45058.
Scherz-Shouval R, Weidberg H, Gonen C, Wilder S, Elazar Z, Oren M . p53-dependent regulation of autophagy protein LC3 supports cancer cell survival under prolonged starvation. Proc Natl Acad Sci USA 2010; 107: 18511–18516.
Maycotte P, Gearheart CM, Barnard R, Aryal S, Mulcahy Levy JM, Fosmire SP et al. STAT3-mediated autophagy dependence identifies subtypes of breast cancer where autophagy inhibition can be efficacious. Cancer Res 2014; 74: 2579–2590.
Selvakumaran M, Amaravadi RK, Vasilevskaya IA, O'Dwyer PJ . Autophagy inhibition sensitizes colon cancer cells to antiangiogenic and cytotoxic therapy. Clin Cancer Res 2013; 19: 2995–3007.
Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W et al. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res 2011; 17: 654–666.
Bristol ML, Emery SM, Maycotte P, Thorburn A, Chakradeo S, Gewirtz DA . Autophagy inhibition for chemosensitization and radiosensitization in cancer: do the preclinical data support this therapeutic strategy? J Pharmacol Exp Ther 2013; 344: 544–552.
Pampaloni F, Reynaud EG, Stelzer EH . The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 2007; 8: 839–845.
Bissell MJ, Weaver VM, Lelievre SA, Wang F, Petersen OW, Schmeichel KL . Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res 1999; 59: 1757–1763s discussion 1763s-1764s.
Edick MJ, Tesfay L, Lamb LE, Knudsen BS, Miranti CK . Inhibition of integrin-mediated crosstalk with epidermal growth factor receptor/Erk or Src signaling pathways in autophagic prostate epithelial cells induces caspase-independent death. Mol Biol Cell 2007; 18: 2481–2490.
Tuloup-Minguez V, Greffard A, Codogno P, Botti J . Regulation of autophagy by extracellular matrix glycoproteins in HeLa cells. Autophagy 2011; 7: 27–39.
Novaro V, Roskelley CD, Bissell MJ . Collagen-IV and laminin-1 regulate estrogen receptor alpha expression and function in mouse mammary epithelial cells. J Cell Sci 2003; 116: 2975–2986.
Debnath J . Detachment-induced autophagy during anoikis and lumen formation in epithelial acini. Autophagy 2008; 4: 351–353.
Lock R, Debnath J . Extracellular matrix regulation of autophagy. Curr Opin Cell Biol 2008; 20: 583–588.
Fung C, Lock R, Gao S, Salas E, Debnath J . Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol Biol Cell 2008; 19: 797–806.
Crighton D, Wilkinson S, Ryan KM . DRAM links autophagy to p53 and programmed cell death. Autophagy 2007; 3: 72–74.
Velez JM, Miriyala S, Nithipongvanitch R, Noel T, Plabplueng CD, Oberley T et al. p53 Regulates oxidative stress-mediated retrograde signaling: a novel mechanism for chemotherapy-induced cardiac injury. PLoS One 2011; 6: e18005.
Jackson JG, Pant V, Li Q, Chang LL, Quintas-Cardama A, Garza D et al. p53-mediated senescence impairs the apoptotic response to chemotherapy and clinical outcome in breast cancer. Cancer Cell 2012; 21: 793–806.
Tasdemir E, Chiara Maiuri M, Morselli E, Criollo A, D'Amelio M, Djavaheri-Mergny M et al. A dual role of p53 in the control of autophagy. Autophagy 2008; 4: 810–814.
Green DR, Kroemer G . Cytoplasmic functions of the tumour suppressor p53. Nature 2009; 458: 1127–1130.
Bhana S, Lloyd DR . The role of p53 in DNA damage-mediated cytotoxicity overrides its ability to regulate nucleotide excision repair in human fibroblasts. Mutagenesis 2008; 23: 43–50.
do Amaral JB, Rezende-Teixeira P, Freitas VM, Machado-Santelli GM . MCF-7 cells as a three-dimensional model for the study of human breast cancer. Tissue Eng Part C Methods 2011; 17: 1097–1107.
Quinet A, Vessoni AT, Rocha CR, Gottifredi V, Biard D, Sarasin A et al. Gap-filling and bypass at the replication fork are both active mechanisms for tolerance of low-dose ultraviolet-induced DNA damage in the human genome. DNA Repair (Amst) 2014; 14: 27–38.
Ghavami S, Mutawe MM, Sharma P, Yeganeh B, McNeill KD, Klonisch T et al. Mevalonate cascade regulation of airway mesenchymal cell autophagy and apoptosis: a dual role for p53. PLoS One 2011; 6: e16523.
Gomes LR, Terra LF, Wailemann RA, Labriola L, Sogayar MC . TGF-beta1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive breast cancer cells. BMC Cancer 2012; 12: 26.
Schmittgen TD, Livak KJ . Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3: 1101–1108.
Acknowledgements
We thank Professor Maria S. Solengas (Department of Dermatology, University of Michigan Comprehensive Cancer Center) for kindly providing the pEGFP-LC3 construct. We are grateful for the excellent technical assistance provided by the Core Facility for Scientific Research-USP (CEFAP-USP) in conducting the confocal fluorescence microscopy assays. This work was supported by FAPESP (Sao Paulo, Brazil), CAPES and CNPq (Brasilia, Brazil).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Gomes, L., Vessoni, A. & Menck, C. Three-dimensional microenvironment confers enhanced sensitivity to doxorubicin by reducing p53-dependent induction of autophagy. Oncogene 34, 5329–5340 (2015). https://doi.org/10.1038/onc.2014.461
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.461
This article is cited by
-
Cytotoxic mechanisms of doxorubicin at clinically relevant concentrations in breast cancer cells
Cancer Chemotherapy and Pharmacology (2022)
-
Multiplexed drug testing of tumor slices using a microfluidic platform
npj Precision Oncology (2020)
-
ATR mediates cisplatin resistance in 3D-cultured breast cancer cells via translesion DNA synthesis modulation
Cell Death & Disease (2019)
-
Elevated hydrostatic pressure enhances the motility and enlarges the size of the lung cancer cells through aquaporin upregulation mediated by caveolin-1 and ERK1/2 signaling
Oncogene (2017)
-
Biomass burning in the Amazon region causes DNA damage and cell death in human lung cells
Scientific Reports (2017)