Abstract
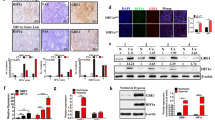
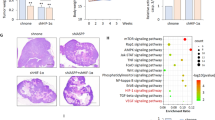
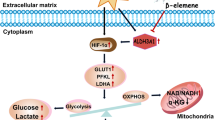
Cancer cells gain a growth advantage through the so-called Warburg effect by shifting glucose metabolism from oxidative phosphorylation to aerobic glycolysis. Hypoxia-inducible factor 1 (HIF-1) has been suggested to function in metabolic reprogramming; however, the underlying mechanism has not been fully elucidated. We found that the aberrant expression of wild-type isocitrate dehydrogenase 3α (IDH3α), a subunit of the IDH3 heterotetramer, decreased α-ketoglutarate levels and increased the stability and transactivation activity of HIF-1α in cancer cells. The silencing of IDH3α significantly delayed tumor growth by suppressing the HIF-1-mediated Warburg effect and angiogenesis. IDH3α expression was associated with the poor postoperative overall survival of lung and breast cancer patients. These results justify the exploitation of IDH3 as a novel target for the diagnosis and treatment of cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Semenza GL . Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003; 3: 721–732.
Semenza GL . Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 2010; 29: 625–634.
Hirota K, Semenza GL . Regulation of hypoxia-inducible factor 1 by prolyl and asparaginyl hydroxylases. Biochem Biophys Res Commun 2005; 338: 610–616.
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001; 292: 468–472.
Mahon PC, Hirota K, Semenza GL . FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev 2001; 15: 2675–2686.
Wang GL, Jiang BH, Rue EA, Semenza GL . Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 1995; 92: 5510–5514.
Semenza GL . Regulation of cancer cell metabolism by hypoxia-inducible factor 1. Semin Cancer Biol 2009; 19: 12–16.
Semenza GL . Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci 2012; 33: 207–214.
Harada H, Kizaka-Kondoh S, Hiraoka M . Optical imaging of tumor hypoxia and evaluation of efficacy of a hypoxia-targeting drug in living animals. Mol Imaging 2005; 4: 182–193.
Shibata T, Akiyama N, Noda M, Sasai K, Hiraoka M . Enhancement of gene expression under hypoxic conditions using fragments of the human vascular endothelial growth factor and the erythropoietin genes. Int J Radiat Oncol Biol Phys 1998; 42: 913–916.
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999; 399: 271–275.
Wakamatsu T, Tanaka T, Oda S, Nishi K, Harada H, Daijo H et al. The intravenous anesthetics barbiturates inhibit hypoxia-inducible factor 1 activation. Eur J Pharmacol 2009; 617: 17–22.
Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL . HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol 2001; 21: 3995–4004.
Mizuno H, Kitada K, Nakai K, Sarai A . PrognoScan: a new database for meta-analysis of the prognostic value of genes. BMC Med Genomics 2009; 2: 18.
Adler E, Euler HV, Gunther G, Plass M . isoCitric dehydrogenase and glutamic acid synthesis in animal tissues. Biochem J 1939; 33: 1028–1045.
McCarthy N . Metabolism: unmasking an oncometabolite. Nat Rev Cancer 2012; 12: 229.
Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 2010; 17: 225–234.
Koivunen P, Lee S, Duncan CG, Lopez G, Lu G, Ramkissoon S et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature 2012; 483: 484–488.
Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL . Transactivation and inhibitory domains of hypoxia-inducible factor 1alpha. Modulation of transcriptional activity by oxygen tension. J Biol Chem 1997; 272: 19253–19260.
Chen C, Okayama H . High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol 1987; 7: 2745–2752.
Harada H, Inoue M, Itasaka S, Hirota K, Morinibu A, Shinomiya K et al. Cancer cells that survive radiation therapy acquire HIF-1 activity and translocate towards tumour blood vessels. Nat Commun 2012; 3: 783.
Harada H, Itasaka S, Kizaka-Kondoh S, Shibuya K, Morinibu A, Shinomiya K et al. The Akt/mTOR pathway assures the synthesis of HIF-1alpha protein in a glucose- and reoxygenation-dependent manner in irradiated tumors. J Biol Chem 2009; 284: 5332–5342.
Harada H, Itasaka S, Zhu Y, Zeng L, Xie X, Morinibu A et al. Treatment regimen determines whether an HIF-1 inhibitor enhances or inhibits the effect of radiation therapy. Br J Cancer 2009; 100: 747–757.
Harada H, Kizaka-Kondoh S, Itasaka S, Shibuya K, Morinibu A, Shinomiya K et al. The combination of hypoxia-response enhancers and an oxygen-dependent proteolytic motif enables real-time imaging of absolute HIF-1 activity in tumor xenografts. Biochem Biophys Res Commun 2007; 360: 791–796.
Harada H, Kizaka-Kondoh S, Li G, Itasaka S, Shibuya K, Inoue M et al. Significance of HIF-1-active cells in angiogenesis and radioresistance. Oncogene 2007; 26: 7508–7516.
Bao Y, Mukai K, Hishiki T, Kubo A, Ohmura M, Sugiura Y et al. Energy management by enhanced glycolysis in G1-phase in human colon cancer cells in vitro and in vivo. Mol Cancer Res 2013; 11: 973–985.
Bajad SU, Lu W, Kimball EH, Yuan J, Peterson C, Rabinowitz JD . Separation and quantitation of water soluble cellular metabolites by hydrophilic interaction chromatography-tandem mass spectrometry. J Chromatogr A 2006; 1125: 76–88.
Zeng L, Ou G, Itasaka S, Harada H, Xie X, Shibuya K et al. TS-1 enhances the effect of radiotherapy by suppressing radiation-induced hypoxia-inducible factor-1 activation and inducing endothelial cell apoptosis. Cancer Sci 2008; 99: 2327–2335.
Tomida S, Takeuchi T, Shimada Y, Arima C, Matsuo K, Mitsudomi T et al. Relapse-related molecular signature in lung adenocarcinomas identifies patients with dismal prognosis. J Clin Oncol 2009; 27: 2793–2799.
Desmedt C, Piette F, Loi S, Wang Y, Lallemand F, Haibe-Kains B et al. Strong time dependence of the 76-gene prognostic signature for node-negative breast cancer patients in the TRANSBIG multicenter independent validation series. Clin Cancer Res 2007; 13: 3207–3214.
Pawitan Y, Bjohle J, Amler L, Borg AL, Egyhazi S, Hall P et al. Gene expression profiling spares early breast cancer patients from adjuvant therapy: derived and validated in two population-based cohorts. Breast Cancer Res 2005; 7: R953–R964.
Acknowledgements
We thank Dr M Suematsu, Dr YA Minamishima, and Dr N Hayakawa for critical discussion. This study was supported by the Funding Program for NEXT Generation World-Leading Researchers (NEXT Program) from the Japan Society for the Promotion of Science (JSPS), Japan to HH (No. LS071), by the program for Precursory Research for Embryonic Science and Technology (PRESTO) from Japan Science and Technology Agency (JST) to HH, by the Project for Development of Innovative Research on Cancer Therapeutics (P-DIRECT) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) to HH, and by Grants-in-Aids for Scientific Research (B) to HH (No. 26293276), for Scientific Research (C) to AM (No. 26461886), for challenging Exploratory Research to HH (No. 26670558) and to MH (No. 26670555), and for Young Scientists (B) to MK (No. 25861088), MY (No. 24791293) and SI (No. 24659563) from MEXT, Japan, by the Sagawa Foundation for the Promotion of Cancer Research to HH, by the Kobayashi Foundation for Cancer Research to HH, by the Takeda Science Foundation to HH, by the Mochida Memorial Foundation for Medical and Pharmaceutical Research to HH, and by the International Science and Technology Cooperation Project of China and Japan to ZL and HH (No. 2010DFA31900).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Zeng, L., Morinibu, A., Kobayashi, M. et al. Aberrant IDH3α expression promotes malignant tumor growth by inducing HIF-1-mediated metabolic reprogramming and angiogenesis. Oncogene 34, 4758–4766 (2015). https://doi.org/10.1038/onc.2014.411
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.411
This article is cited by
-
γ-Glutamylcyclotransferase, a novel regulator of HIF-1α expression, triggers aerobic glycolysis
Cancer Gene Therapy (2022)
-
Transcriptomic and metabolomic characterization of post-hatch metabolic reprogramming during hepatic development in the chicken
BMC Genomics (2021)
-
The role of hypoxia in the tumor microenvironment and development of cancer stem cell: a novel approach to developing treatment
Cancer Cell International (2021)
-
Isoforms of IDH in breast carcinoma: IDH2 as a potent prognostic factor associated with proliferation in estrogen-receptor positive cases
Breast Cancer (2021)
-
The different expression of glycogen phosphorylases in renal clear cell renal carcinoma and chromophobe renal carcinoma
Clinical Proteomics (2020)