Abstract
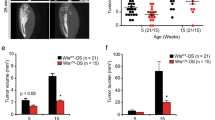
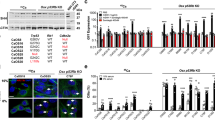
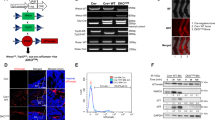
Osteosarcomas remain an enigmatic group of malignancies that share in common the presence of transformed cells producing osteoid matrix, even if these cells comprise a minority of the tumor volume. The differentiation state of osteosarcomas has therefore become a topic of interest and challenge to those who study this disease. In order to test how the cell of origin contributes to the final state of differentiation in the transformed cells, we compared the relative tumorigenicity of Cre-LoxP conditional disruption of the cell cycle checkpoint tumor-suppressor genes Trp53 and Rb1 using Prx1-Cre, Collagen-1α1-Cre and Osteocalcin-Cre to transform undifferentiated mesenchyme, preosteoblasts and mature osteoblasts, respectively. The Prx1 and Col1α1 lineages developed tumors with nearly complete penetrance, as anticipated. Osteosarcomas also developed in 44% of Oc-Cre;Rb1fl/fl;Trp53fl/fl mice. We confirmed using 5-ethynyl-2′-deoxyuridine click chemistry that the Oc-Cre lineage includes very few actively cycling cells. By assessing radiographic mineralization and histological osteoid production, the differentiation state of tumors did not correlate with the differentiation state of the lineage of origin. Some of the osteocalcin-lineage-derived osteosarcomas were among the least osteoblastic. Osteocalcin immunohistochemistry in tumors correlated well with the expression of DNA methyl transferases, suggesting that silencing of these epigenetic regulators may influence the final differentiation state of an osteosarcoma. Transformation of differentiated, minimally proliferative osteoblasts is possible but may require such an epigenetic reprogramming that the tumors no longer resemble their differentiated origins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dick JE . Breast cancer stem cells revealed. Proc Natl Acad Sci USA 2003; 100: 3547–3549.
Reya T, Morrison SJ, Clarke MF, Weissman IL . Stem cells, cancer, and cancer stem cells. Nature 2001; 414: 105–111.
Sharkey FE . Morphometric analysis of differentiation in human breast carcinoma. Tumor heterogeneity. Arch Pathol Lab Med 1983; 107: 411–414.
Meacham CE, Morrison SJ . Tumour heterogeneity and cancer cell plasticity. Nature 2013; 501: 328–337.
Angus-Hill ML, Elbert KM, Hidalgo J, Capecchi MR . T-cell factor 4 functions as a tumor suppressor whose disruption modulates colon cell proliferation and tumorigenesis. Proc Natl Acad Sci USA 2011; 108: 4914–4919.
Liu F, Malaval L, Gupta AK, Aubin JE . Simultaneous detection of multiple bone-related mRNAs and protein expression during osteoblast differentiation: polymerase chain reaction and immunocytochemical studies at the single cell level. Dev Biol 1994; 166: 220–234.
Owen TA, Holthuis J, Markose E, van Wijnen AJ, Wolfe SA, Grimes SR et al. Modifications of protein-DNA interactions in the proximal promoter of a cell-growth-regulated histone gene during onset and progression of osteoblast differentiation. Proc Natl Acad Sci USA 1990; 87: 5129–5133.
Gibbs CP, Kukekov VG, Reith JD, Tchigrinova O, Suslov ON, Scott EW et al. Stem-like cells in bone sarcomas: implications for tumorigenesis. Neoplasia 2005; 7: 967–976.
Wu C, Wei Q, Utomo V, Nadesan P, Whetstone H, Kandel R et al. Side population cells isolated from mesenchymal neoplasms have tumor initiating potential. Cancer Res 2007; 67: 8216–8222.
Levings PP, McGarry SV, Currie TP, Nickerson DM, McClellan S, Ghivizzani SC et al. Expression of an exogenous human Oct-4 promoter identifies tumor-initiating cells in osteosarcoma. Cancer Res 2009; 69: 5648–5655.
Damron TA, Ward WG, Stewart A . Osteosarcoma, chondrosarcoma, and Ewing's sarcoma: National Cancer Data Base Report. Clin Orthop Relat Res 2007; 459: 40–47.
Desandes E . Survival from adolescent cancer. Cancer Treat Rev 2007; 33: 609–615.
Unni KK, Dahlin DC . Osteosarcoma: pathology and classification. Semin Roentgenol 1989; 24: 143–152.
Mutsaers AJ, Walkley CR . Cells of origin in osteosarcoma: mesenchymal stem cells or osteoblast committed cells? Bone 2014; 62C: 56–63.
Amary MF, Bacsi K, Maggiani F, Damato S, Halai D, Berisha F et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol 2011; 224: 334–343.
Ragland BD, Bell WC, Lopez RR, Siegal GP . Cytogenetics and molecular biology of osteosarcoma. Lab Invest 2002; 82: 365–373.
Weiss A, Khoury JD, Hoffer FA, Wu J, Billups CA, Heck RK et al. Telangiectatic osteosarcoma: the St. Jude Children's Research Hospital's experience. Cancer 2007; 109: 1627–1637.
Hopyan S, Gokgoz N, Bell RS, Andrulis IL, Alman BA, Wunder JS . Expression of osteocalcin and its transcriptional regulators core-binding factor alpha 1 and MSX2 in osteoid-forming tumours. J Orthop Res 1999; 17: 633–638.
Thomas DM, Carty SA, Piscopo DM, Lee JS, Wang WF, Forrester WC et al. The retinoblastoma protein acts as a transcriptional coactivator required for osteogenic differentiation. Mol Cell 2001; 8: 303–316.
Thomas DM, Johnson SA, Sims NA, Trivett MK, Slavin JL, Rubin BP et al. Terminal osteoblast differentiation, mediated by runx2 and p27KIP1, is disrupted in osteosarcoma. J Cell Biol 2004; 167: 925–934.
Gutierrez GM, Kong E, Sabbagh Y, Brown NE, Lee JS, Demay MB et al. Impaired bone development and increased mesenchymal progenitor cells in calvaria of RB1−/− mice. Proc Natl Acad Sci USA 2008; 105: 18402–18407.
Lengner CJ, Steinman HA, Gagnon J, Smith TW, Henderson JE, Kream BE et al. Osteoblast differentiation and skeletal development are regulated by Mdm2-p53 signaling. J Cell Biol 2006; 172: 909–921.
Wang X, Kua HY, Hu Y, Guo K, Zeng Q, Wu Q et al. p53 functions as a negative regulator of osteoblastogenesis, osteoblast-dependent osteoclastogenesis, and bone remodeling. J Cell Biol 2006; 172: 115–125.
Berman SD, Calo E, Landman AS, Danielian PS, Miller ES, West JC et al. Metastatic osteosarcoma induced by inactivation of Rb and p53 in the osteoblast lineage. Proc Natl Acad Sci USA 2008; 105: 11851–11856.
Lin PP, Pandey MK, Jin F, Raymond AK, Akiyama H, Lozano G . Targeted mutation of p53 and Rb in mesenchymal cells of the limb bud produces sarcomas in mice. Carcinogenesis 2009; 30: 1789–1795.
San Martin IA, Varela N, Gaete M, Villegas K, Osorio M, Tapia JC et al. Impaired cell cycle regulation of the osteoblast-related heterodimeric transcription factor Runx2-Cbfbeta in osteosarcoma cells. J Cell Physiol 2009; 221: 560–571.
Walkley CR, Shea JM, Sims NA, Purton LE, Orkin SH . Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell 2007; 129: 1081–1095.
Walkley CR, Qudsi R, Sankaran VG, Perry JA, Gostissa M, Roth SI et al. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev 2008; 22: 1662–1676.
Molyneux SD, Di Grappa MA, Beristain AG, McKee TD, Wai DH, Paderova J et al. Prkar1a is an osteosarcoma tumor suppressor that defines a molecular subclass in mice. J Clin Invest 2010; 120: 3310–3325.
Lin PP, Pandey MK, Jin F, Xiong S, Deavers M, Parant JM et al. EWS-FLI1 induces developmental abnormalities and accelerates sarcoma formation in a transgenic mouse model. Cancer Res 2008; 68: 8968–8975.
Dacquin R, Starbuck M, Schinke T, Karsenty G . Mouse alpha1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev Dyn 2002; 224: 245–251.
Liu F, Woitge HW, Braut A, Kronenberg MS, Lichtler AC, Mina M et al. Expression and activity of osteoblast-targeted Cre recombinase transgenes in murine skeletal tissues. Int J Dev Biol 2004; 48: 645–653.
Rossert J, Eberspaecher H, de Crombrugghe B . Separate cis-acting DNA elements of the mouse pro-alpha 1(I) collagen promoter direct expression of reporter genes to different type I collagen-producing cells in transgenic mice. J Cell Biol 1995; 129: 1421–1432.
Yeo H, Beck LH, Thompson SR, Farach-Carson MC, McDonald JM, Clemens TL et al. Conditional disruption of calcineurin B1 in osteoblasts increases bone formation and reduces bone resorption. J Biol Chem 2007; 282: 35318–35327.
Haydon RC, Zhou L, Feng T, Breyer B, Cheng H, Jiang W et al. Nuclear receptor agonists as potential differentiation therapy agents for human osteosarcoma. Clin Cancer Res 2002; 8: 1288–1294.
He BC, Chen L, Zuo GW, Zhang W, Bi Y, Huang J et al. Synergistic antitumor effect of the activated PPARgamma and retinoid receptors on human osteosarcoma. Clin Cancer Res 2010; 16: 2235–2245.
Acknowledgements
We thank Thomas Clemens for the Oc-Cre mouse line, Malcolm Logan for Prx1-Cre and Benoit de Crombrugghe for Col1α1-Cre. This work was directly supported by National Cancer Institute (National Institutes of Health, NIH) K08CA138764. KBJ receives additional career development support from the Damon Runyon Cancer Research Foundation. This work was also partly supported by P30CA042014 from the National Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Quist, T., Jin, H., Zhu, JF. et al. The impact of osteoblastic differentiation on osteosarcomagenesis in the mouse. Oncogene 34, 4278–4284 (2015). https://doi.org/10.1038/onc.2014.354
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.354
This article is cited by
-
PRRX1-TOP2A interaction is a malignancy-promoting factor in human malignant peripheral nerve sheath tumours
British Journal of Cancer (2024)
-
FOXP1 drives osteosarcoma development by repressing P21 and RB transcription downstream of P53
Oncogene (2021)
-
Molecular mechanisms underpinning sarcomas and implications for current and future therapy
Signal Transduction and Targeted Therapy (2021)
-
Rho-GEF Trio regulates osteosarcoma progression and osteogenic differentiation through Rac1 and RhoA
Cell Death & Disease (2021)
-
Induction and expansion of human PRRX1+ limb-bud-like mesenchymal cells from pluripotent stem cells
Nature Biomedical Engineering (2021)