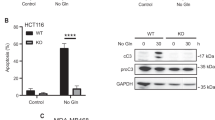
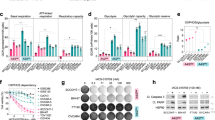
Abstract
Cellular transformation is associated with altered glutamine (Gln) metabolism. Tumor cells utilize Gln in the tricarboxylic acid (TCA) cycle to maintain sufficient pools of biosynthetic precursors to support rapid growth and proliferation. However, Gln metabolism also generates NADPH, and Gln-derived glutamate is used for synthesis of glutathione (GSH). As both NADPH and GSH are antioxidants, Gln may also contribute to redox balance in transformed cells. The Hace1 E3 ligase is a tumor suppressor inactivated in diverse human cancers. Hace1 targets the Rac1 GTPase for degradation at Rac1-dependent NADPH oxidase complexes, blocking superoxide generation by the latter. Consequently, loss of Hace1 increases reactive oxygen species (ROS) levels in vitro and in vivo. Given the link between Hace1 loss and increased ROS, we investigated whether genetic inactivation of Hace1 alters Gln metabolism. We demonstrate that mouse embryonic fibroblasts (MEFs) derived from Hace1−/− mice are highly sensitive to Gln withdrawal, leading to enhanced cell death compared with wild-type (wt) MEFs, and Gln depletion or chemical inhibition of Gln uptake blocks soft agar colony formation by Hace1−/− MEFs. Hace1−/− MEFs exhibit increased Gln uptake and ammonia secretion, and metabolic labeling using 13C-Gln revealed that Hace1 loss increases incorporation of Gln carbons into the TCA cycle intermediates. Gln starvation markedly increases ROS levels in Hace1−/− but not in wt MEFs, and treatment with the antioxidant N-acetyl cysteine or the TCA cycle intermediate oxaloacetate efficiently rescues Gln starvation-induced ROS elevation and cell death in Hace1−/− MEFs. Finally, Gln starvation increases superoxide levels in Hace1−/− MEFs, and NADPH oxidase inhibitors block the induction of superoxide and cell death by Gln starvation. Together, these results suggest that increased ROS production due to Hace1 loss leads to Gln addiction as a mechanism to cope with increased ROS-induced oxidative stress.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Anglesio MS, Evdokimova V, Melnyk N, Zhang L, Fernandez CV, Grundy PE et al. Differential expression of a novel ankyrin containing E3 ubiquitin-protein ligase, Hace1, in sporadic Wilms' tumor versus normal kidney. Hum Mol Genet 2004; 13: 2061–2074.
Zhang L, Anglesio MS, O'Sullivan M, Zhang F, Yang G, Sarao R et al. The E3 ligase HACE1 is a critical chromosome 6q21 tumor suppressor involved in multiple cancers. Nat Med 2007; 13: 1060–1069.
Hibi K, Sakata M, Sakuraba K, Shirahata A, Goto T, Mizukami H et al. Aberrant methylation of the HACE1 gene is frequently detected in advanced colorectal cancer. Anticancer Res 2008; 28: 1581–1584.
Thelander EF, Ichimura K, Corcoran M, Barbany G, Nordgren A, Heyman M et al. Characterization of 6q deletions in mature B cell lymphomas and childhood acute lymphoblastic leukemia. Leuk Lymphoma 2008; 49: 477–487.
Sakata M, Kitamura YH, Sakuraba K, Goto T, Mizukami H, Saito M et al. Methylation of HACE1 in gastric carcinoma. Anticancer Res 2009; 29: 2231–2233.
Huang Y, de Reynies A, de Leval L, Ghazi B, Martin-Garcia N, Travert M et al. Gene expression profiling identifies emerging oncogenic pathways operating in extranodal NK/T-cell lymphoma, nasal type. Blood 2010; 115: 1226–1237.
Slade I, Stephens P, Douglas J, Barker K, Stebbings L, Abbaszadeh F et al. Constitutional translocation breakpoint mapping by genome-wide paired-end sequencing identifies HACE1 as a putative Wilms tumour susceptibility gene. J Med Genet 2010; 47: 342–347.
Diskin SJ, Capasso M, Schnepp RW, Cole KA, Attiyeh EF, Hou C et al. Common variation at 6q16 within HACE1 and LIN28B influences susceptibility to neuroblastoma. Nat Genet 2012; 44: 1126–1130.
Torrino S, Visvikis O, Doye A, Boyer L, Stefani C, Munro P et al. The E3 ubiquitin-ligase HACE1 catalyzes the ubiquitylation of active Rac1. Dev Cell 2011; 21: 959–965.
Castillo-Lluva S, Tan CT, Daugaard M, Sorensen PH, Malliri A . The tumour suppressor HACE1 controls cell migration by regulating Rac1 degradation. Oncogene 2013; 32: 1735–1742.
Cheng G, Diebold BA, Hughes Y, Lambeth JD . Nox1-dependent reactive oxygen generation is regulated by Rac1. J Biol Chem 2006; 281: 17718–17726.
Ueyama T, Geiszt M, Leto TL . Involvement of Rac1 in activation of multicomponent Nox1- and Nox3-based NADPH oxidases. Mol Cell Biol 2006; 26: 2160–2174.
Daugaard M, Nitsch R, Razaghi B, McDonald L, Jarrar A, Torrino S et al. Hace1 controls ROS generation of vertebrate Rac1-dependent NADPH oxidase complexes. Nat Commun 2013; 4: 2180.
Rotblat B, Southwell AL, Ehrnhoefer DE, Skotte NH, Metzler M, Franciosi S et al. HACE1 reduces oxidative stress and mutant Huntingtin toxicity by promoting the NRF2 response. Proc Natl Acad Sci USA 2014; 111: 3032–3037.
Cantor JR, Sabatini DM . Cancer cell metabolism: one hallmark, many faces. Cancer Discov 2012; 2: 881–898.
DeBerardinis RJ . Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genet Med 2008; 10: 767–777.
Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB . Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev 2008; 18: 54–61.
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB . The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 2008; 7: 11–20.
Klimberg VS, McClellan JL . Claude H. Organ Jr. Honorary Lectureship. Glutamine, cancer, and its therapy. Am J Surg 1996; 172: 418–424.
Rajagopalan KN, DeBerardinis RJ . Role of glutamine in cancer: therapeutic and imaging implications. J Nucl Med. 2011; 52: 1005–1008.
Wise DR, Thompson CB . Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci 2010; 35: 427–433.
Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA 2008; 105: 18782–18787.
Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 2009; 458: 762–765.
Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA 2010; 107: 8788–8793.
Reynolds MR, Lane AN, Robertson B, Kemp S, Liu Y, Hill BG et al. Control of glutamine metabolism by the tumor suppressor Rb. Oncogene 2013; 33: 556–566.
Nicolay BN, Gameiro PA, Tschop K, Korenjak M, Heilmann AM, Asara JM et al. Loss of RBF1 changes glutamine catabolism. Genes Dev 2013; 27: 182–196.
Shanware NP, Mullen AR, DeBerardinis RJ, Abraham RT . Glutamine: pleiotropic roles in tumor growth and stress resistance. J Mol Med (Berl) 2011; 89: 229–236.
DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA 2007; 104: 19345–19350.
DeBerardinis RJ, Cheng T . Q's next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010; 29: 313–324.
Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD . Quantitative flux analysis reveals folate-dependent NADPH production. Nature 2014; 510: 298–302.
Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 2013; 496: 101–105.
Wellen KE, Lu C, Mancuso A, Lemons JM, Ryczko M, Dennis JW et al. The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev 2010; 24: 2784–2799.
Tong X, Zhao F, Thompson CB . The molecular determinants of de novo nucleotide biosynthesis in cancer cells. Curr Opin Genet Dev 2009; 19: 32–37.
Moldave K, Meister A . Synthesis of phenylacetylglutamine by human tissue. J Biol Chem 1957; 229: 463–476.
Li XN, Parikh S, Shu Q, Jung HL, Chow CW, Perlaky L et al. Phenylbutyrate and phenylacetate induce differentiation and inhibit proliferation of human medulloblastoma cells. Clin Cancer Res 2004; 10: 1150–1159.
Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 2009; 136: 521–534.
Gianni D, Taulet N, Zhang H, DerMardirossian C, Kister J, Martinez L et al. A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem Biol 2010; 5: 981–993.
Cheng T, Sudderth J, Yang C, Mullen AR, Jin ES, Mates JM et al. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proc Natl Acad Sci USA 2011; 108: 8674–8679.
Fernandez CA, Des Rosiers C, Previs SF, David F, Brunengraber H . Correction of 13C mass isotopomer distributions for natural stable isotope abundance. J Mass Spectrom 1996; 31: 255–262.
Acknowledgements
This research was supported by funds from the Canadian Institute of Health Research (MOP-123416) and British Columbia Cancer Foundation through generous donations from Team Finn and Ride to Conquer Cancer (to PHS), and from the National Institutes of Health (R01 CA157996) and Damon-Runyon Cancer Research Foundation (to RJD). NC was funded by the Canadian Institutes of Health Research, Frederick Banting and Charles Best Canada Graduate Scholarship Doctoral Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Cetinbas, N., Daugaard, M., Mullen, A. et al. Loss of the tumor suppressor Hace1 leads to ROS-dependent glutamine addiction. Oncogene 34, 4005–4010 (2015). https://doi.org/10.1038/onc.2014.316
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.316
This article is cited by
-
HACE1 negatively regulates neuroinflammation through ubiquitylating and degrading Rac1 in Parkinson’s disease models
Acta Pharmacologica Sinica (2022)
-
Postsynaptic p47phox regulates long-term depression in the hippocampus
Cell Discovery (2018)
-
Identification of cancer-associated missense mutations in hace1 that impair cell growth control and Rac1 ubiquitylation
Scientific Reports (2017)
-
Arginine dependence of tumor cells: targeting a chink in cancer’s armor
Oncogene (2016)
-
ROS homeostasis and metabolism: a dangerous liason in cancer cells
Cell Death & Disease (2016)