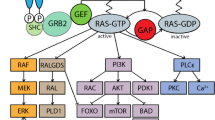
Abstract
The Pim protein kinases contribute to transformation by enhancing the activity of oncogenic Myc and Ras, which drives significant metabolic changes during tumorigenesis. In this report, we demonstrate that mouse embryo fibroblasts (MEFs) lacking all three isoforms of Pim protein kinases, triple knockout (TKO), cannot tolerate the expression of activated K-Ras (K-RasG12V) and undergo cell death. Transduction of K-RasG12V into these cells markedly increased the level of cellular reactive oxygen species (ROS). The addition of N-acetyl cysteine attenuated ROS production and reversed the cytotoxic effects of K-RasG12V in the TKO MEFs. The altered cellular redox state caused by the loss of Pim occurred as a result of lower levels of metabolic intermediates in the glycolytic and pentose phosphate pathways as well as abnormal mitochondrial oxidative phosphorylation. TKO MEFs exhibit reduced levels of superoxide dismutase (Sod), glutathione peroxidase 4 (Gpx4) and peroxiredoxin 3 (Prdx3) that render them susceptible to killing by K-RasG12V-mediated ROS production. In contrast, the transduction of c-Myc into TKO cells can overcome the lack of Pim protein kinases by regulating cellular metabolism and Sod2. In the absence of the Pim kinases, c-Myc transduction permitted K-RasG12V-induced cell growth by decreasing Ras-induced cellular ROS levels. These results demonstrate that the Pim protein kinases have an important role in regulating cellular redox, metabolism and K-Ras-stimulated cell growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Verbeek S, van Lohuizen M, van der Valk M, Domen J, Kraal G, Berns A . Mice bearing the E mu-myc and E mu-pim-1 transgenes develop pre-B-cell leukemia prenatally. Mol Cell Biol 1991; 11: 1176–1179.
van Lohuizen M, Verbeek S, Krimpenfort P, Domen J, Saris C, Radaszkiewicz T et al. Predisposition to lymphomagenesis in pim-1 transgenic mice: cooperation with c-myc and N-myc in murine leukemia virus-induced tumors. Cell 1989; 56: 673–682.
Adam M, Pogacic V, Bendit M, Chappuis R, Nawijn MC, Duyster J et al. Targeting PIM kinases impairs survival of hematopoietic cells transformed by kinase inhibitor-sensitive and kinase inhibitor-resistant forms of Fms-like tyrosine kinase 3 and BCR/ABL. Cancer Res 2006; 66: 3828–3835.
Agrawal S, Koschmieder S, Baumer N, Reddy NG, Berdel WE, Muller-Tidow C et al. Pim2 complements Flt3 wild-type receptor in hematopoietic progenitor cell transformation. Leukemia 2008; 22: 78–86.
Kim KT, Baird K, Ahn JY, Meltzer P, Lilly M, Levis M et al. Pim-1 is up-regulated by constitutively activated FLT3 and plays a role in FLT3-mediated cell survival. Blood 2005; 105: 1759–1767.
Wang J, Anderson PD, Luo W, Gius D, Roh M, Abdulkadir SA . Pim1 kinase is required to maintain tumorigenicity in MYC-expressing prostate cancer cells. Oncogene 2012; 31: 1794–1803.
Wang JB, Erickson JW, Fuji R, Ramachandran S, Gao P, Dinavahi R et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell 2010; 18 207–219.
Beharry Z, Mahajan S, Zemskova M, Lin YW, Tholanikunnel BG, Xia Z et al. The Pim protein kinases regulate energy metabolism and cell growth. Proc Natl Acad Sci USA 2011; 108: 528–533.
Soucek L, Whitfield JR, Sodir NM, Masso-Valles D, Serrano E, Karnezis AN et al. Inhibition of Myc family proteins eradicates KRas-driven lung cancer in mice. Genes Dev 2013; 27: 504–513.
Tran PT, Fan AC, Bendapudi PK, Koh S, Komatsubara K, Chen J et al. Combined Inactivation of MYC and K-Ras oncogenes reverses tumorigenesis in lung adenocarcinomas and lymphomas. PLoS ONE 2008; 3: e2125.
Compere SJ, Baldacci P, Sharpe AH, Thompson T, Land H, Jaenisch R . The ras and myc oncogenes cooperate in tumor induction in many tissues when introduced into midgestation mouse embryos by retroviral vectors. Proc Natl Acad Sci USA 1989; 86: 2224–2228.
Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012; 149: 656–670.
Gaglio D, Metallo CM, Gameiro PA, Hiller K, Danna LS, Balestrieri C et al. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol Syst Biol 2011; 7: 523.
Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 2013; 496: 101–105.
Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA 2010; 107: 8788–8793.
Mitsushita J, Lambeth JD, Kamata T . The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation. Cancer Res 2004; 64: 3580–3585.
Osthus RC, Shim H, Kim S, Li Q, Reddy R, Mukherjee M et al. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem 2000; 275: 21797–21800.
Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 2009; 458: 762–765.
Liu W, Le A, Hancock C, Lane AN, Dang CV, Fan TW et al. Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC. Proc Natl Acad Sci USA 2012; 109: 8983–8988.
Menssen A, Hydbring P, Kapelle K, Vervoorts J, Diebold J, Luscher B et al. The c-MYC oncoprotein, the NAMPT enzyme, the SIRT1-inhibitor DBC1, and the SIRT1 deacetylase form a positive feedback loop. Proc Natl Acad Sci USA 2012; 109: E187–E196.
DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 2011; 475: 106–109.
Wang X, Magnuson S, Pastor R, Fan E, Hu H, Tsui V et al. Discovery of novel pyrazolo[1,5-a]pyrimidines as potent pan-Pim inhibitors by structure- and property-based drug design. Bioorg Med Chem Lett 2013; 23: 3149–3153.
Lu J, Zavorotinskaya T, Dai Y, Niu XH, Castillo J, Sim J et al. Pim2 is required for maintaining multiple myeloma cell growth through modulating TSC2 phosphorylation. Blood 2013; 122: 1610–1620.
Garcia PD, Langowski JL, Wang Y, Chen M, Castillo J, Fanton C et al. Pan-PIM kinase inhibition provides a novel therapy for treating hematologic cancers. Clin Cancer Res 2014; 20: 1834–1845.
Gomez-Duran A, Pacheu-Grau D, Martinez-Romero I, Lopez-Gallardo E, Lopez-Perez MJ, Montoya J et al. Oxidative phosphorylation differences between mitochondrial DNA haplogroups modify the risk of Leber's hereditary optic neuropathy. Biochim Biophys Acta 2012; 1822: 1216–1222.
Bhalla K, Hwang BJ, Dewi RE, Ou L, Twaddel W, Fang HB et al. PGC1alpha promotes tumor growth by inducing gene expression programs supporting lipogenesis. Cancer Res 2011; 71: 6888–6898.
Ziegler M . New functions of a long-known molecule. Emerging roles of NAD in cellular signaling. Eur J Biochem 2000; 267: 1550–1564.
Santidrian AF, Matsuno-Yagi A, Ritland M, Seo BB, LeBoeuf SE, Gay LJ et al. Mitochondrial complex I activity and NAD+/NADH balance regulate breast cancer progression. J Clin Invest 2013; 123: 1068–1081.
Gorrini C, Harris IS, Mak TW . Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 2013; 12: 931–947.
Gaetani GF, Galiano S, Canepa L, Ferraris AM, Kirkman HN . Catalase and glutathione peroxidase are equally active in detoxification of hydrogen peroxide in human erythrocytes. Blood 1989; 73: 334–339.
Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014; 156: 317–331.
Podsypanina K, Politi K, Beverly LJ, Varmus HE . Oncogene cooperation in tumor maintenance and tumor recurrence in mouse mammary tumors induced by Myc and mutant Kras. Proc Natl Acad Sci USA 2008; 105: 5242–5247.
Sinn E, Muller W, Pattengale P, Tepler I, Wallace R, Leder P . Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo. Cell 1987; 49: 465–475.
Li F, Wang Y, Zeller KI, Potter JJ, Wonsey DR, O'Donnell KA et al. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol Cell Biol 2005; 25: 6225–6234.
de Groof AJ, te Lindert MM, van Dommelen MM, Wu M, Willemse M, Smift AL et al. Increased OXPHOS activity precedes rise in glycolytic rate in H-RasV12/E1A transformed fibroblasts that develop a Warburg phenotype. Mol Cancer 2009; 8: 54.
Telang S, Lane AN, Nelson KK, Arumugam S, Chesney J . The oncoprotein H-RasV12 increases mitochondrial metabolism. Mol Cancer 2007; 6: 77.
Pitkanen S, Robinson BH . Mitochondrial complex I deficiency leads to increased production of superoxide radicals and induction of superoxide dismutase. J Clin Invest 1996; 98: 345–351.
Diaz F, Garcia S, Padgett KR, Moraes CT . A defect in the mitochondrial complex III, but not complex IV, triggers early ROS-dependent damage in defined brain regions. Hum Mol Genet 2012; 21: 5066–5077.
Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 2013; 342: 1243417.
Mikkers H, Nawijn M, Allen J, Brouwers C, Verhoeven E, Jonkers J et al. Mice deficient for all PIM kinases display reduced body size and impaired responses to hematopoietic growth factors. Mol Cell Biol 2004; 24: 6104–6115.
Cai H, Memarzadeh S, Stoyanova T, Beharry Z, Kraft AS, Witte ON . Collaboration of KRas and androgen receptor signaling stimulates EZH2 expression and tumor-propagating cells in prostate cancer. Cancer Res 2012; 72: 4672–4681.
Song JH, Kraft AS . Pim kinase inhibitors sensitize prostate cancer cells to apoptosis triggered by Bcl-2 family inhibitor ABT-737. Cancer Res 2012; 72: 294–303.
Song JH, Bellail A, Tse MC, Yong VW, Hao C . Human astrocytes are resistant to Fas ligand and tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. J Neurosci 2006; 26: 3299–3308.
Langley RJ, Tsalik EL, van Velkinburgh JC, Glickman SW, Rice BJ, Wang C et al. An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci Transl Med 2013; 5: 195ra95.
Weiner J 3rd, Parida SK, Maertzdorf J, Black GF, Repsilber D, Telaar A et al. Biomarkers of inflammation, immunosuppression and stress with active disease are revealed by metabolomic profiling of tuberculosis patients. PLoS ONE 2012; 7: e40221.
Acknowledgements
This work was supported in part by the Genomics Shared Resource at Hollings Cancer Center and Seahorse Biosciences Academic Core Facility, Medical University of South Carolina. We thank Dr Allen J Ebens at Genentech Inc. and Novartis Oncology for providing the Pim kinase inhibitors used in this study. This work was supported by the NIH P30-CA138313, DOD W81XWH-08-PCRP-IDA, R01 CA1732000, and American Cancer Society Institutional Research Grant awarded to the Hollings Cancer Center, Medical University of South Carolina.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Song, J., An, N., Chatterjee, S. et al. Deletion of Pim kinases elevates the cellular levels of reactive oxygen species and sensitizes to K-Ras-induced cell killing. Oncogene 34, 3728–3736 (2015). https://doi.org/10.1038/onc.2014.306
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.306
This article is cited by
-
Phosphorylation of PFKFB4 by PIM2 promotes anaerobic glycolysis and cell proliferation in endometriosis
Cell Death & Disease (2022)
-
PIM kinases inhibit AMPK activation and promote tumorigenicity by phosphorylating LKB1
Cell Communication and Signaling (2021)
-
PIM kinases alter mitochondrial dynamics and chemosensitivity in lung cancer
Oncogene (2020)
-
Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis
Nature Chemical Biology (2016)