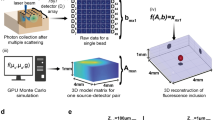
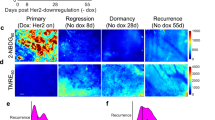
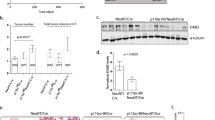
Abstract
Human epidermal growth factor receptor2/Neu, which is overexpressed in about 30% of human breast cancers, transduces growth signals in large part via the Ras–Raf–MEK–ERK pathway. Nevertheless, it is a matter of controversy whether high ERK activity in breast cancer tissues correlates with better or worse prognosis, leaving the role of ERK activity in the progression of breast cancers unresolved. To address this issue, we live-imaged ERK activity in mammary tumors developed in mouse mammary tumor virus-Neu transgenic mice, which had been crossed with transgenic mice expressing a Förster resonance energy transfer biosensor for ERK. Observation of the tumor by two-photon microscopy revealed significant heterogeneity in ERK activity among the mammary tumor cells. The level of ERK activity in each cell was stable up to several hours, implying a robust mechanism that maintained the ERK activity within a limited range. By sorting the mammary tumor cells on the basis of their ERK activity, we found that ERKhigh cells less efficiently generated tumorspheres in vitro and tumors in vivo than did ERKlow cells. In agreement with this finding, the expressions of the cancer stem cell markers CD49f, CD24 and CD61 were decreased in ERKhigh cells. These observations suggest that high ERK activity may suppress the self-renewal of mammary cancer stem cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Visvader JE, Lindeman GJ . Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 2008; 8: 755–768.
Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB . Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov 2009; 8: 806–823.
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989; 244: 707–712.
Magnifico A, Albano L, Campaner S, Delia D, Castiglioni F, Gasparini P et al. Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to trastuzumab. Clin Cancer Res 2009; 15: 2010–2021.
Korkaya H, Paulson A, Iovino F, Wicha MS . HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene 2008; 27: 6120–6130.
Chang CJ, Yang JY, Xia W, Chen CT, Xie X, Chao CH et al. EZH2 promotes expansion of breast tumor initiating cellsthrough activation of RAF1-beta-catenin signaling. Cancer Cell 2011; 19: 86–100.
Whyte J, Bergin O, Bianchi A, McNally S, Martin F . Key signalling nodes in mammary gland development and cancer. Mitogen-activated protein kinase signalling in experimental models of breast cancer progression and in mammary gland development. Breast Cancer Res 2009; 11: 209.
Guy CTWM, Schaller M, Parsons TJ, Cardiff RD, Muller WJ . Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA 1992; 89: 10578–10582.
Ursini-Siegel J, Schade B, Cardiff RD, Muller WJ . Insights from transgenic mouse models of ERBB2-induced breast cancer. Nat Rev Cancer 2007; 7: 389–397.
Jeselsohn R, Brown NE, Arendt L, Klebba I, Hu MG, Kuperwasser C et al. Cyclin D1 kinase activity is required for the self-renewal of mammary stem and progenitor cells that are targets of MMTV-ErbB2 tumorigenesis. Cancer Cell 2010; 17: 65–76.
Izrailit J, Berman HK, Datti A, Wrana JL, Reedijk M . High throughput kinase inhibitor screens reveal TRB3 and MAPK-ERK/TGFbeta pathways as fundamental Notch regulators in breast cancer. Proc Natl Acad Sci USA 2013; 110: 1714–1719.
Mueller H, Flury N, Eppenberger-Castori S, Kueng W, David F, Eppenberger U . Potential prognostic value of mitogen-activated protein kinase activityfor disease-free survival of primary breast cancer patients. Int J Cancer 2000; 89: 384–388.
McGlynn LM, Kirkegaard T, Edwards J, Tovey S, Cameron D, Twelves C et al. Ras/Raf-1/MAPK pathway mediates response to tamoxifen but not chemotherapy in breast cancer patients. Clin Cancer Res 2009; 15: 1487–1495.
Bergqvist J, Elmberger G, Ohd J, Linderholm B, Bjohle J, Hellborg H et al. Activated ERK1/2 and phosphorylated oestrogen receptor alpha are associated with improved breast cancer survival in women treated with tamoxifen. Eur J Cancer 2006; 42: 1104–1112.
Svensson S, Jirstrom K, Ryden L, Roos G, Emdin S, Ostrowski MC et al. ERK phosphorylation is linked to VEGFR2 expression and Ets-2 phosphorylation in breast cancer and is associated with tamoxifen treatment resistance and small tumours with good prognosis. Oncogene 2005; 24: 4370–4379.
Milde-Langosch K, Bamberger AM, Rieck G, Grund D, Hemminger G, Muller V et al. Expression and prognostic relevance of activated extracellular-regulated kinases (ERK1/2) in breast cancer. Br J Cancer 2005; 92: 2206–2215.
Kunath T, Saba-El-Leil MK, Almousailleakh M, Wray J, Meloche S, Smith A . FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development 2007; 134: 2895–2902.
Chappell J, Sun Y, Singh A, Dalton S . MYC/MAX control ERK signaling and pluripotency by regulation of dual-specificity phosphatases 2 and 7. Genes Dev 2013; 27: 725–733.
Greber B, Wu G, Bernemann C, Joo JY, Han DW, Ko K et al. Conserved and divergent roles of FGF signaling in mouse epiblast stem cells and human embryonic stem cells. Cell Stem Cell 2010; 6: 215–226.
Goto A, Sumiyama K, Kamioka Y, Nakasyo E, Ito K, Iwasaki M et al. GDNF and endothelin 3 regulate migration of enteric neural crest-derived cells via protein kinase A and Rac1. J Neurosci 2013; 33: 4901–4912.
Kamioka Y, Sumiyama K, Mizuno R, Sakai Y, Hirata E, Kiyokawa E et al. Live imaging of protein kinase activities in transgenic mice expressing FRET biosensors. Cell Struct Funct 2012; 37: 65–73.
Nobis M, McGhee EJ, Morton JP, Schwarz JP, Karim SA, Quinn J et al. Intravital FLIM-FRET Imaging reveals dasatinib-induced spatial control of src in pancreatic cancer. Cancer Res 2013; 73: 4674–4686.
Brown AP, Carlson TC, Loi CM, Graziano MJ . Pharmacodynamic and toxicokinetic evaluation of the novel MEK inhibitor, PD0325901, in the rat following oral and intravenous administration. Cancer Chemother Pharmacol 2007; 59: 671–679.
Liu L, Greger J, Shi H, Liu Y, Greshock J, Annan R et al. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res 2009; 69: 6871–6878.
Liu JC, Voisin V, Bader GD, Deng T, Pusztai L, Symmans WF et al. Seventeen-gene signature from enriched Her2/Neu mammary tumor-initiating cells predicts clinical outcome for human HER2+:ERα- breast cancer. Proc Natl Acad Sci USA 2012; 109: 5832–5837.
Lo PK, Kanojia D, Liu X, Singh UP, Berger FG, Wang Q et al. CD49f and CD61 identify Her2/neu-induced mammary tumor-initiating cells that are potentially derived from luminal progenitors and maintained by the integrin-TGFbeta signaling. Oncogene 2012; 31: 2614–2626.
Montesano R, Soulie P . Retinoids induce lumen morphogenesis in mammary epithelial cells. J Cell Sci 2002; 115: 4419–4431.
Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell 2010; 140: 62–73.
Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D et al. Purification and unique properties of mammary epithelial stem cells. Nature 2006; 439: 993–997.
Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML et al. Generation of a functional mammary gland from a single stem cell. Nature 2006; 439: 84–88.
Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol 2007; 9: 201–209.
Vaillant F, Asselin-Labat ML, Shackleton M, Forrest NC, Lindeman GJ, Visvader JE . The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cellsin mouse models of mammary tumorigenesis. Cancer Res 2008; 68: 7711–7717.
Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J et al. The ground state of embryonic stem cell self-renewal. Nature 2008; 453: 519–523.
Parashurama N, Lobo NA, Ito K, Mosley AR, Habte FG, Zabala M et al. Remodeling of endogenous mammary epithelium by breast cancer stem cells. Stem Cells 2012; 30: 2114–2127.
Aoki K, Matsuda M . Visualization of small GTPase activity with fluorescence resonance energy transfer-based biosensors. Nat Protoc 2009; 4: 1623–1631.
Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z . Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell 2008; 14: 570–581.
Acknowledgements
J3B1 cells were kindly provided by Roberto Montesano. We thank Y Inaoka, K Hirano, K Takakura, R Tabata, Y Kitagawa and A Kawagishi for their technical assistance. We are grateful to the members of the Matsuda Laboratory for their helpful discussions. MM was supported by a Grant-in-Aid for Scientific Research on the Innovative Area of ‘Fluorescence Live imaging’ (No. 22113002) of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), and by an Innovative Techno-Hub for the Integrated Medical Bio-imaging Project of the Special Coordination Funds for Promoting Science and Technology by MEXT, Japan. YK was supported by a Grant-in-Aid for JSPS Fellows from MEXT, Japan. The animal protocols were reviewed and approved by the Animal Care and Use Committee of Kyoto University Graduate School of Medicine (No. 10584).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Kumagai, Y., Naoki, H., Nakasyo, E. et al. Heterogeneity in ERK activity as visualized by in vivo FRET imaging of mammary tumor cells developed in MMTV-Neu mice. Oncogene 34, 1051–1057 (2015). https://doi.org/10.1038/onc.2014.28
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.28
This article is cited by
-
A cell-autonomous tumour suppressor role of RAF1 in hepatocarcinogenesis
Nature Communications (2016)
-
ERK1/2 is related to oestrogen receptor and predicts outcome in hormone-treated breast cancer
Breast Cancer Research and Treatment (2014)