Abstract
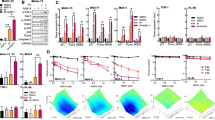
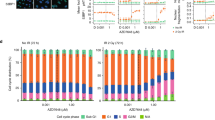
Topoisomerase inhibitors are in common use as chemotherapeutic agents although they can display reduced efficacy in chemotherapy-resistant tumours, which have inactivated DNA damage response (DDR) genes, such as ATM and TP53. Here, we characterise the cellular response to the dual-acting agent, Alchemix (ALX), which is a modified anthraquinone that functions as a topoisomerase inhibitor as well as an alkylating agent. We show that ALX induces a robust DDR at nano-molar concentrations and this is mediated primarily through ATR- and DNA-PK- but not ATM-dependent pathways, despite DNA double strand breaks being generated after prolonged exposure to the drug. Interestingly, exposure of epithelial tumour cell lines to ALX in vitro resulted in potent activation of the G2/M checkpoint, which after a prolonged arrest, was bypassed allowing cells to progress into mitosis where they ultimately died by mitotic catastrophe. We also observed effective killing of lymphoid tumour cell lines in vitro following exposure to ALX, although, in contrast, this tended to occur via activation of a p53-independent apoptotic pathway. Lastly, we validate the effectiveness of ALX as a chemotherapeutic agent in vivo by demonstrating its ability to cause a significant reduction in tumour cell growth, irrespective of TP53 status, using a mouse leukaemia xenograft model. Taken together, these data demonstrate that ALX, through its dual action as an alkylating agent and topoisomerase inhibitor, represents a novel anti-cancer agent that could be potentially used clinically to treat refractory or relapsed tumours, particularly those harbouring mutations in DDR genes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Nitiss JL Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer 2009; 9: 338–350.
Nitiss JL DNA topoisomerase II and its growing repertoire of biological functions. Nat Rev Cancer 2009; 9: 327–337.
Larsen AK, Escargueil AE, Skladanowski A Catalytic topoisomerase II inhibitors in cancer therapy. Pharmacol Ther 2003; 99: 167–181.
Pommier Y Drugging topoisomerases: lessons and challenges. ACS Chem Biol 2013; 8: 82–95.
Wang JC Cellular roles of DNA topoisomerases: a molecular perspective. Nat Rev Mol Cell Biol 2002; 3: 430–440.
Beeharry N, Rattner JB, Caviston JP, Yen T Centromere fragmentation is a common mitotic defect of S and G2 checkpoint override. Cell Cycle 2013; 12: 1588–1597.
Al-Ejeh F, Kumar R, Wiegmans A, Lakhani SR, Brown MP, Khanna KK Harnessing the complexity of DNA-damage response pathways to improve cancer treatment outcomes. Oncogene 2010; 29: 6085–6098.
Longley DB, Johnston PG Molecular mechanisms of drug resistance. J Pathol 2005; 205: 275–292.
Pors K, Paniwnyk Z, Teesdale-Spittle P, Plumb JA, Willmore E, Austin CA et al. Alchemix: a novel alkylating anthraquinone with potent activity against anthracycline- and cisplatin-resistant ovarian cancer. Mol Cancer Ther 2003; 2: 607–610.
Pors K, Paniwnyk Z, Ruparelia KC, Teesdale-Spittle PH, Hartley JA, Kelland LR et al. Synthesis and biological evaluation of novel chloroethylaminoanthraquinones with potent cytotoxic activity against cisplatin-resistant tumor cells. J Med Chem 2004; 47: 1856–1859.
Abdallah QM, Phillips RM, Johansson F, Helleday T, Cosentino L, Abdel-Rahman H et al. Minor structural modifications to alchemix influence mechanism of action and pharmacological activity. Biochem Pharmacol 2012; 83: 1514–1522.
Caldecott K, Banks G, Jeggo P DNA double-strand break repair pathways and cellular tolerance to inhibitors of topoisomerase II. Cancer Res 1990; 50: 5778–5783.
Forrest RA, Swift LP, Evison BJ, Rephaeli A, Nudelman A, Phillips DR et al. The hydroxyl epimer of doxorubicin controls the rate of formation of cytotoxic anthracycline-DNA adducts. Cancer Chemother Pharmacol 2013; 71: 809–816.
Ward IM, Chen J Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem 2001; 276: 47759–47762.
Yajima H, Lee KJ, Chen BP ATR-dependent phosphorylation of DNA-dependent protein kinase catalytic subunit in response to UV-induced replication stress. Mol Cell Biol 2006; 26: 7520–7528.
Yajima H, Lee KJ, Zhang S, Kobayashi J, Chen BP DNA double-strand break formation upon UV-induced replication stress activates ATM and DNA-PKcs kinases. J Mol Biol 2009; 385: 800–810.
McNeely S, Conti C, Sheikh T, Patel H, Zabludoff S, Pommier Y et al. Chk1 inhibition after replicative stress activates a double strand break response mediated by ATM and DNA-dependent protein kinase. Cell Cycle 2010; 9: 995–1004.
Wang Y, Qin J . MSH2 and ATR form a signaling module and regulate two branches of the damage response to DNA methylation. Proc Natl Acad Sci USA 2003; 100: 15387–15392.
Pabla N, Ma Z, McIlhatton MA, Fishel R, Dong Z hMSH2 recruits ATR to DNA damage sites for activation during DNA damage-induced apoptosis. J Biol Chem 2011; 286: 10411–10418.
Pors K, Patterson LH DNA mismatch repair deficiency, resistance to cancer chemotherapy and the development of hypersensitive agents. Curr Top Med Chem 2005; 5: 1133–1149.
Pors K, Plumb JA, Brown R, Teesdale-Spittle P, Searcey M, Smith PJ et al. Development of nonsymmetrical 1, 4-disubstituted anthraquinones that are potently active against cisplatin-resistant ovarian cancer cells. J Med Chem 2005; 48: 6690–6695.
Sy SM, Jiang J, Dong SS, Lok GT, Wu J, Cai H et al. Critical roles of ring finger protein RNF8 in replication stress responses. J Biol Chem 2011; 286: 22355–22361.
Orth JD, Loewer A, Lahav G, Mitchison TJ Prolonged mitotic arrest triggers partial activation of apoptosis, resulting in DNA damage and p53 induction. Mol Biol Cell 2012; 23: 567–576.
Bester AC, Roniger M, Oren YS, Im MM, Sarni D, Chaoat M et al. Nucleotide deficiency promotes genomic instability in early stages of cancer development. Cell 2011; 145: 435–446.
Jones RM, Mortusewicz O, Afzal I, Lorvellec M, García P, Helleday T et al. Increased replication initiation and conflicts with transcription underlie Cyclin E-induced replication stress. Oncogene 2013; 32: 3744–3753.
Stankovic T, Weber P, Stewart G, Bedenham T, Murray J, Byrd PJ et al. Inactivation of ataxia telangiectasia mutated gene in B-cell chronic lymphocytic leukaemia. Lancet 1999; 353: 26–29.
Vorechovský I, Luo L, Dyer MJ, Catovsky D, Amlot PL, Yaxley JC et al. Clustering of missense mutations in the ataxia-telangiectasia gene in a sporadic T-cell leukaemia. Nat Genet 1997; 17: 96–99.
Schaffner C, Idler I, Stilgenbauer S, Döhner H, Lichter P Mantle cell lymphoma is characterized by inactivation of the ATM gene. Proc Natl Acad Sci USA 2000; 97: 2773–2778.
Bhojwani D, Pui CH Relapsed childhood acute lymphoblastic leukaemia. Lancet Oncol 2013; 14: e205–e217.
Inaba H, Greaves M, Mullighan CG Acute lymphoblastic leukaemia. Lancet 2013; 81: 1943–1955.
Hof J, Krentz S, van Schewick C, Körner G, Shalapour S, Rhein P et al. Mutations and deletions of the TP53 gene predict nonresponse to treatment and poor outcome in first relapse of childhood acute lymphoblastic leukemia. J Clin Oncol 2011; 29: 3185–3193.
Holmfeldt L, Wei L, Diaz-Flores E, Walsh M, Zhang J, Ding L et al. The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nat Genet 2013; 45: 242–252.
Chan EY, Kir S, Tooze SA siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J Biol Chem 2007; 282: 25464–25474.
Greaves M Return of the malingering mutants. Br J Cancer 2013; 109: 1391–1393.
Redmond KM, Wilson TR, Johnston PG, Longley DB Resistance mechanisms to cancer chemotherapy. Front Biosci 2008; 13: 5138–5154.
Stewart GS, Panier S, Townsend K, Al-Hakim AK, Kolas NK, Miller ES et al. The RIDDLE syndrome protein mediates a ubiquitin-dependent signaling cascade at sites of DNA damage. Cell 2009; 136: 420–434.
Petermann E, Orta ML, Issaeva N, Schultz N, Helleday T Hydroxyurea-stalled replication forks become progressively inactivated and require two different RAD51-mediated pathways for restart and repair. Mol Cell 2010; 37: 492–502.
Acknowledgements
We are most grateful to Fred Bunz, Alan D’Andrea, Eva Petermann, Panagiotis Kotsantis and Jiri Lukas for the gift of cell lines, and Philip Byrd, Arnold Levine and David Lane for antibodies. We would especially like to thank Cyrus Vaziri for providing cell lines and a recombinant adenovirus expressing Cyclin E, Rebbeca Jones for helping us with PFGE and Malcolm Taylor for helpful discussions. Last, we would also thank the University of Birmingham (AT—PhD studentship), MRC (GSS, RJG—Project grant: G0900088), the Cancer Research UK (GSS—Senior Fellowship: C17183/A13030), the Leukaemia Lymphoma Research (TS, GSS—Programme grant: 11045), the Lister Institute (GSS, AZ—Research Prize) and the Yorkshire Cancer Research (KP) for funding this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Thomas, A., Perry, T., Berhane, S. et al. The dual-acting chemotherapeutic agent Alchemix induces cell death independently of ATM and p53. Oncogene 34, 3336–3348 (2015). https://doi.org/10.1038/onc.2014.266
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.266
This article is cited by
-
Mitoxantrone and Analogues Bind and Stabilize i-Motif Forming DNA Sequences
Scientific Reports (2016)