Abstract
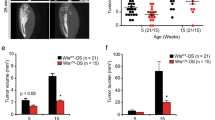
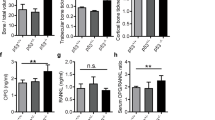
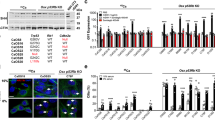
Osteosarcoma (OS) is the most common cancer of bone. Parathyroid hormone (PTH) regulates calcium homeostasis and bone development, while the paracrine/autocrine PTH-related protein (PTHrP) has central roles in endochondral bone formation and bone remodeling. Using a murine OS model, we found that OS cells express PTHrP and the common PTH/PTHrP receptor (PTHR1). To investigate the role of PTHR1 signaling in OS cell behavior, we used shRNA to reduce PTHR1 expression. This only mildly inhibited proliferation in vitro, but markedly reduced invasion through collagen and reduced expression of RANK ligand (RANKL). Administration of PTH(1–34) did not stimulate OS proliferation in vivo but, strikingly, PTHR1 knockdown resulted in a profound growth inhibition and increased differentiation/mineralization of the tumors. Treatment with neutralizing antibody to PTHrP did not recapitulate the knockdown of PTHR1. Consistent with this lack of activity, PTHrP was predominantly intracellular in OS cells. Knockdown of PTHR1 resulted in increased expression of late osteoblast differentiation genes and upregulation of Wnt antagonists. RANKL production was reduced in knockdown tumors, providing for reduced homotypic signaling through the receptor, RANK. Loss of PTHR1 resulted in the coordinated loss of gene signatures associated with the polycomb repressive complex 2 (PRC2). Using Ezh2 inhibitors, we demonstrate that the increased expression of osteoblast maturation markers is in part mediated by the loss of PRC2 activity. Collectively these results demonstrate that PTHR1 signaling is important in maintaining OS proliferation and undifferentiated state. This is in part mediated by intracellular PTHrP and through regulation of the OS epigenome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Allison DC, Carney SC, Ahlmann ER, Hendifar A, Chawla S, Fedenko A et al. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma 2012; 2012: 704872.
Janeway KA, Grier HE . Sequelae of osteosarcoma medical therapy: a review of rare acute toxicities and late effects. Lancet Oncol 2010; 11: 670–678.
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. New Engl J Med 2001; 344: 1434–1441.
McCauley LK, Martin TJ . Twenty-five years of PTHrP progress: from cancer hormone to multifunctional cytokine. J Bone Miner Res 2012; 27: 1231–1239.
Gardella TJ, Juppner H . Molecular properties of the PTH/PTHrP receptor. Trends Endocrinol Metab 2001; 12: 210–217.
Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC . Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest 1999; 104: 439–446.
Keller H, Kneissel M . SOST is a target gene for PTH in bone. Bone 2005; 37: 148–158.
Andrews EB, Gilsenan AW, Midkiff K, Sherrill B, Wu Y, Mann BH et al. The US postmarketing surveillance study of adult osteosarcoma and teriparatide: study design and findings from the first 7 years. J Bone Miner Res 2012; 27: 2429–2437.
Tashjian AH Jr, Goltzman D . On the interpretation of rat carcinogenicity studies for human PTH(1-34) and human PTH(1-84). J Bone Miner Res 2008; 23: 803–811.
Yang R, Hoang BH, Kubo T, Kawano H, Chou A, Sowers R et al. Over-expression of parathyroid hormone Type 1 receptor confers an aggressive phenotype in osteosarcoma. Int J Cancer 2007; 121: 943–954.
Martin TJ, Ingleton PM, Underwood JC, Michelangeli VP, Hunt NH, Melick RA . Parathyroid hormone-responsive adenylate cyclase in induced transplantable osteogenic rat sarcoma. Nature 1976; 260: 436–438.
Majeska RJ, Rodan SB, Rodan GA . Parathyroid hormone-responsive clonal cell lines from rat osteosarcoma. Endocrinology 1980; 107: 1494–1503.
Ingleton PM, Underwood JC, Hunt NH, Atkins D, Giles B, Coulton LA et al. Radiation induced osteogenic sarcoma in the rat as a model of hormone-responsive differentiated cancer. Lab Anim Sci 1977; 27 (5 Pt 2): 748–756.
Walkley CR, Qudsi R, Sankaran VG, Perry JA, Gostissa M, Roth SI et al. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev 2008; 22: 1662–1676.
Mutsaers AJ, Ng AJ, Baker EK, Russell MR, Chalk AM, Wall M et al. Modeling distinct osteosarcoma subtypes in vivo using Cre:lox and lineage-restricted transgenic shRNA. Bone 2013; 55: 166–178.
Kuijjer ML, Peterse EF, van den Akker BE, Briaire-de Bruijn IH, Serra M, Meza-Zepeda LA et al. IR/IGF1R signaling as potential target for treatment of high-grade osteosarcoma. BMC Cancer 2013; 13: 245.
Kuijjer ML, Namlos HM, Hauben EI, Machado I, Kresse SH, Serra M et al. mRNA expression profiles of primary high-grade central osteosarcoma are preserved in cell lines and xenografts. BMC Med Genomics 2011; 4: 66.
Allan EH, Ho PW, Umezawa A, Hata J, Makishima F, Gillespie MT et al. Differentiation potential of a mouse bone marrow stromal cell line. J Cell Biochem 2003; 90: 158–169.
Takiguchi M, Dow LE, Prier JE, Carmichael CL, Kile BT, Turner SJ et al. Variability of inducible expression across the hematopoietic system of tetracycline transactivator transgenic mice. PLoS One 2013; 8: e54009.
Premsrirut PK, Dow LE, Kim SY, Camiolo M, Malone CD, Miething C et al. A rapid and scalable system for studying gene function in mice using conditional RNA interference. Cell 2011; 145: 145–158.
Molyneux SD, Di Grappa MA, Beristain AG, McKee TD, Wai DH, Paderova J et al. Prkar1a is an osteosarcoma tumor suppressor that defines a molecular subclass in mice. J Clin Invest 2010; 120: 3310–3325.
Thompson DL, Sabbagh Y, Tenenhouse HS, Roche PC, Drezner MK, Salisbury JL et al. Ontogeny of Phex/PHEX protein expression in mouse embryo and subcellular localization in osteoblasts. J Bone Miner Res 2002; 17: 311–320.
Allan EH, Hausler KD, Wei T, Gooi JH, Quinn JM, Crimeen-Irwin B et al. EphrinB2 regulation by PTH and PTHrP revealed by molecular profiling in differentiating osteoblasts. J Bone Miner Res 2008; 23: 1170–1181.
Miao D, He B, Karaplis AC, Goltzman D . Parathyroid hormone is essential for normal fetal bone formation. J Clin Invest 2002; 109: 1173–1182.
Martin TJ . Osteoblast-derived PTHrP is a physiological regulator of bone formation. J Clin Invest 2005; 115: 2322–2324.
Lam MH, Olsen SL, Rankin WA, Ho PW, Martin TJ, Gillespie MT et al. PTHrP and cell division: expression and localization of PTHrP in a keratinocyte cell line (HaCaT) during the cell cycle. J Cell Physiol 1997; 173: 433–446.
Danks JA, Ebeling PR, Hayman J, Chou ST, Moseley JM, Dunlop J et al. Parathyroid hormone-related protein: immunohistochemical localization in cancers and in normal skin. J Bone Miner Res 1989; 4: 273–278.
Onuma E, Sato K, Saito H, Tsunenari T, Ishii K, Esaki K et al. Generation of a humanized monoclonal antibody against human parathyroid hormone-related protein and its efficacy against humoral hypercalcemia of malignancy. Anticancer Res 2004; 24: 2665–2673.
Mundy GR . Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002; 2: 584–593.
Lamoureux F, Richard P, Wittrant Y, Battaglia S, Pilet P, Trichet V et al. Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: blockade of the vicious cycle between tumor cell proliferation and bone resorption. Cancer Res 2007; 67: 7308–7318.
Akiyama T, Choong PF, Dass CR . RANK-Fc inhibits malignancy via inhibiting ERK activation and evoking caspase-3-mediated anoikis in human osteosarcoma cells. Clin Exp Metastasis 2010; 27: 207–215.
Gorlick R, Janeway K, Lessnick S, Randall RL, Marina N . Children's Oncology Group's 2013 blueprint for research: bone tumors. Pediatr Blood Cancer 2013; 60: 1009–1015.
Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 2006; 440: 692–696.
Beristain AG, Narala SR, Di Grappa MA, Khokha R . Homotypic RANK signaling differentially regulates proliferation, motility and cell survival in osteosarcoma and mammary epithelial cells. J Cell Sci 2012; 125 (Pt 4): 943–955.
Vargas MA, St-Louis M, Desgroseillers L, Charli JL, Boileau G . Parathyroid hormone-related protein(1-34) regulates Phex expression in osteoblasts through the protein kinase A pathway. Endocrinology 2003; 144: 4876–4885.
Gopalakrishnan R, Suttamanatwong S, Carlson AE, Franceschi RT . Role of matrix Gla protein in parathyroid hormone inhibition of osteoblast mineralization. Cells Tissues Organs 2005; 181: 166–175.
Henderson JE, Amizuka N, Warshawsky H, Biasotto D, Lanske BM, Goltzman D et al. Nucleolar localization of parathyroid hormone-related peptide enhances survival of chondrocytes under conditions that promote apoptotic cell death. Mol Cell Biol 1995; 15: 4064–4075.
Massfelder T, Dann P, Wu TL, Vasavada R, Helwig JJ, Stewart AF . Opposing mitogenic and anti-mitogenic actions of parathyroid hormone-related protein in vascular smooth muscle cells: a critical role for nuclear targeting. Proc Natl Acad Sci USA 1997; 94: 13630–13635.
Jiang B, Morimoto S, Fukuo K, Yasuda O, Chen S, Ogihara T . Role of parathyroid hormone-related protein in the proliferation of vascular smooth muscle cells. Miner Electrolyte Metab 1995; 21: 157–160.
Maeda S, Wu S, Juppner H, Green J, Aragay AM, Fagin JA et al. Cell-specific signal transduction of parathyroid hormone (PTH)-related protein through stably expressed recombinant PTH/PTHrP receptors in vascular smooth muscle cells. Endocrinology 1996; 137: 3154–3162.
Pirola CJ, Wang HM, Kamyar A, Wu S, Enomoto H, Sharifi B et al. Angiotensin II regulates parathyroid hormone-related protein expression in cultured rat aortic smooth muscle cells through transcriptional and post-transcriptional mechanisms. J Biol Chem 1993; 268: 1987–1994.
Okano K, Wu S, Huang X, Pirola CJ, Juppner H, Abou-Samra AB et al. Parathyroid hormone (PTH)/PTH-related protein (PTHrP) receptor and its messenger ribonucleic acid in rat aortic vascular smooth muscle cells and UMR osteoblast-like cells: cell-specific regulation by angiotensin-II and PTHrP. Endocrinology 1994; 135: 1093–1099.
Jans DA, Briggs LJ, Gustin SE, Jans P, Ford S, Young IG . The cytokine interleukin-5 (IL-5) effects cotransport of its receptor subunits to the nucleus in vitro. FEBS Lett 1997; 410: 368–372.
Liu Y, Berendsen AD, Jia S, Lotinun S, Baron R, Ferrara N et al. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest 2012; 122: 3101–3113.
Watson PH, Fraher LJ, Hendy GN, Chung UI, Kisiel M, Natale BV et al. Nuclear localization of the type 1 PTH/PTHrP receptor in rat tissues. J Bone Miner Res 2000; 15: 1033–1044.
Dickins RA, Hemann MT, Zilfou JT, Simpson DR, Ibarra I, Hannon GJ et al. Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nat Genet 2005; 37: 1289–1295.
Acknowledgements
We thank the SVH BioResources Centre; P Kocovski and S Taylor for technical assistance, L Purton and J Heierhorst for comments and discussion. This work was supported by grants from the National Health and Medical Research Council of Australia (NHMRC; to CRW and CRW/TJM); Cancer Council of Victoria (to CRW and EKB); NHMRC Career Development Award (CRW); Cure Cancer Australia Foundation Fellowship (EKB); in part by the Victorian State Government Operational Infrastructure Support Program (to St. Vincent’s Institute). CRW is the Philip Desbrow Senior Research Fellow of the Leukaemia Foundation. The authors thank Chugai Pharmaceutical Company for providing the α-PTHrP neutralizing antibody; The Structural Genomics Consortium for generously providing GSK343 and UNC1999.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Ho, P., Goradia, A., Russell, M. et al. Knockdown of PTHR1 in osteosarcoma cells decreases invasion and growth and increases tumor differentiation in vivo. Oncogene 34, 2922–2933 (2015). https://doi.org/10.1038/onc.2014.217
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.217
This article is cited by
-
Parathyroid hormone-related peptide and parathyroid hormone-related peptide receptor type 1 in locally advanced laryngeal cancer as prognostic indicators of relapse and survival
BMC Cancer (2022)
-
Mangiferin suppresses human metastatic osteosarcoma cell growth by down-regulating the expression of metalloproteinases-1/2 and parathyroid hormone receptor 1
AMB Express (2020)
-
Parathyroid hormone receptor 1 (PTHR1) is a prognostic indicator in canine osteosarcoma
Scientific Reports (2020)
-
Smac mimetics LCL161 and GDC-0152 inhibit osteosarcoma growth and metastasis in mice
BMC Cancer (2019)
-
Osteosarcoma in the Post Genome Era: Preclinical Models and Approaches to Identify Tractable Therapeutic Targets
Current Osteoporosis Reports (2019)