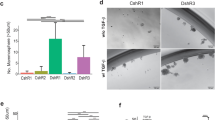
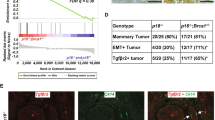
Abstract
Disease progression and recurrence are major barriers to survival for breast cancer patients. Understanding the etiology of recurrent or metastatic breast cancer and underlying mechanisms is critical for the development of new treatments and improved survival. Here, we report that two commonly overexpressed breast cancer oncogenes, Ron (Recepteur d’Origine Nantaise) and DEK, cooperate to promote advanced disease through multipronged effects on β-catenin signaling. The Ron receptor is commonly activated in breast cancers, and Ron overexpression in human disease stimulates β-catenin nuclear translocation and is an independent predictor of metastatic dissemination. Dek is a chromatin-associated oncogene whose expression has been linked to cancer through multiple mechanisms, including β-catenin activity. We demonstrate here that Dek is a downstream target of Ron receptor activation in murine and human models. The absence of Dek in the MMTV-Ron mouse model led to a significant delay in tumor development, characterized by decreased cell proliferation, diminished metastasis and fewer cells expressing mammary cancer stem cell markers. Dek complementation of cell lines established from this model was sufficient to promote cellular growth and invasion. Mechanistically, Dek expression stimulated the production and secretion of Wnt ligands to sustain an autocrine/paracrine canonical β-catenin signaling loop. Finally, we show that Dek overexpression promotes tumorigenic phenotypes in immortalized human mammary epithelial MCF10A cells and, in the context of Ron receptor activation, correlates with disease recurrence and metastasis in patients. Overall, our studies demonstrate that DEK overexpression, due in part to Ron receptor activation, drives breast cancer progression through the induction of Wnt/β-catenin signaling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
ACS. Breast Cancer Facts & Figures 2011–2012. American Cancer Society: Atlanta, GA, USA, 2011.
Maggiora P, Marchio S, Stella MC, Giai M, Belfiore A, De Bortoli M et al. Overexpression of the RON gene in human breast carcinoma. Oncogene 1998; 16: 2927–2933.
Zinser GM, Leonis MA, Toney K, Pathrose P, Thobe M, Kader SA et al. Mammary-specific Ron receptor overexpression induces highly metastatic mammary tumors associated with beta-catenin activation. Cancer Res 2006; 66: 11967–11974.
Zhao H, Chen MS, Lo YH, Waltz SE, Wang J, Ho PC et al. The Ron receptor tyrosine kinase activates c-Abl to promote cell proliferation through tyrosine phosphorylation of PCNA in breast cancer. Oncogene 2013; 33: 1429–1437.
Danilkovitch-Miagkova A . Oncogenic signaling pathways activated by RON receptor tyrosine kinase. Curr Cancer Drug Targets 2003; 3: 31–40.
Wagh PK, Gray JK, Zinser GM, Vasiliauskas J, James L, Monga SP et al. Beta-catenin is required for Ron receptor-induced mammary tumorigenesis. Oncogene 2011; 30: 3694–3704.
Leonis MA, Thobe MN, Waltz SE . Ron-receptor tyrosine kinase in tumorigenesis and metastasis. Fut Oncol 2007; 3: 441–448.
Privette Vinnedge LM, Kappes F, Nassar N, Wells SI . Stacking the DEK: from chromatin topology to cancer stem cells. Cell Cycle 2013; 12: 51–66.
Alexiadis V, Waldmann T, Andersen J, Mann M, Knippers R, Gruss C . The protein encoded by the proto-oncogene DEK changes the topology of chromatin and reduces the efficiency of DNA replication in a chromatin-specific manner. Genes Dev 2000; 14: 1308–1312.
Campillos M, Garcia MA, Valdivieso F, Vazquez J . Transcriptional activation by AP-2alpha is modulated by the oncogene DEK. Nucleic Acids Res 2003; 31: 1571–1575.
McGarvey T, Rosonina E, McCracken S, Li Q, Arnaout R, Mientjes E et al. The acute myeloid leukemia-associated protein, DEK, forms a splicing-dependent interaction with exon–product complexes. J Cell Biol 2000; 150: 309–320.
Soares LM, Zanier K, Mackereth C, Sattler M, Valcarcel J . Intron removal requires proofreading of U2AF/3' splice site recognition by DEK. Science 2006; 312: 1961–1965.
Kavanaugh GM, Wise-Draper TM, Morreale RJ, Morrison MA, Gole B, Schwemberger S et al. The human DEK oncogene regulates DNA damage response signaling and repair. Nucleic Acids Res 2011; 39: 7465–7476.
Lossaint G, Larroque M, Ribeyre C, Bec N, Larroque C, Decaillet C et al. FANCD2 binds MCM proteins and controls replisome function upon activation of S phase checkpoint signaling. Mol Cell 2013; 51: 678–690.
Kappes F, Waldmann T, Mathew V, Yu J, Zhang L, Khodadoust MS et al. The DEK oncoprotein is a Su(var) that is essential to heterochromatin integrity. Genes Dev 2011; 25: 673–678.
Sawatsubashi S, Murata T, Lim J, Fujiki R, Ito S, Suzuki E et al. A histone chaperone, DEK, transcriptionally coactivates a nuclear receptor. Genes Dev 2010; 24: 159–170.
Kim DW, Kim JY, Choi S, Rhee S, Hahn Y, Seo SB . Transcriptional regulation of 1-cys peroxiredoxin by the proto-oncogene protein DEK. Mol Med Rep 2010; 3: 877–881.
Liu K, Feng T, Liu J, Zhong M, Zhang S . Silencing of the DEK gene induces apoptosis and senescence in CaSki cervical carcinoma cells via the up-regulation of NF-kappaB p65. Biosci Rep 2012; 32: 323–332.
Sammons M, Wan SS, Vogel NL, Mientjes EJ, Grosveld G, Ashburner BP . Negative regulation of the RelA/p65 transactivation function by the product of the DEK proto-oncogene. J Biol Chem 2006; 281: 26802–26812.
Karam M, Thenoz M, Capraro V, Robin JP, Pinatel C, Lancon A et al. Chromatin Redistribution of the DEK oncoprotein represses hTERT transcription in leukemias. Neoplasia 2014; 16: 21–30.
Gamble MJ, Fisher RP . SET and PARP1 remove DEK from chromatin to permit access by the transcription machinery. Nat Struct Mol Biol 2007; 14: 548–555.
Koleva RI, Ficarro SB, Radomska HS, Carrasco-Alfonso MJ, Alberta JA, Webber JT et al. C/EBPalpha and DEK coordinately regulate myeloid differentiation. Blood 2012; 119: 4878–4888.
Mor-Vaknin N, Kappes F, Dick AE, Legendre M, Damoc C, Teitz-Tennenbaum S et al. DEK in the synovium of patients with juvenile idiopathic arthritis: characterization of DEK antibodies and posttranslational modification of the DEK autoantigen. Arthritis Rheum 2011; 63: 556–567.
Mor-Vaknin N, Punturieri A, Sitwala K, Faulkner N, Legendre M, Khodadoust MS et al. The DEK nuclear autoantigen is a secreted chemotactic factor. Mol Cell Biol 2006; 26: 9484–9496.
Datta A, Adelson ME, Mogilevkin Y, Mordechai E, Sidi AA, Trama JP . Oncoprotein DEK as a tissue and urinary biomarker for bladder cancer. BMC Cancer 2011; 11: 234.
Saha AK, Kappes F, Mundade A, Deutzmann A, Rosmarin DM, Legendre M et al. Intercellular trafficking of the nuclear oncoprotein DEK. Proc Natl Acad Sci USA 2013; 110: 6847–6852.
Khodadoust MS, Verhaegen M, Kappes F, Riveiro-Falkenbach E, Cigudosa JC, Kim DS et al. Melanoma proliferation and chemoresistance controlled by the DEK oncogene. Cancer Res 2009; 69: 6405–6413.
Kondoh N, Wakatsuki T, Ryo A, Hada A, Aihara T, Horiuchi S et al. Identification and characterization of genes associated with human hepatocellular carcinogenesis. Cancer Res 1999; 59: 4990–4996.
Privette Vinnedge LM, McClaine R, Wagh PK, Wikenheiser-Brokamp KA, Waltz SE, Wells SI . The human DEK oncogene stimulates beta-catenin signaling, invasion and mammosphere formation in breast cancer. Oncogene 2011; 30: 2741–2752.
Wise-Draper TM, Mintz-Cole RA, Morris TA, Simpson DS, Wikenheiser-Brokamp KA, Currier MA et al. Overexpression of the cellular DEK protein promotes epithelial transformation in vitro and in vivo. Cancer Res 2009; 69: 1792–1799.
von Lindern M, Fornerod M, van Baal S, Jaegle M, de Wit T, Buijs A et al. The translocation (6;9), associated with a specific subtype of acute myeloid leukemia, results in the fusion of two genes, dek and can, and the expression of a chimeric, leukemia-specific dek-can mRNA. Mol Cell Biol 1992; 12: 1687–1697.
Shibata T, Kokubu A, Miyamoto M, Hosoda F, Gotoh M, Tsuta K et al. DEK oncoprotein regulates transcriptional modifiers and sustains tumor initiation activity in high-grade neuroendocrine carcinoma of the lung. Oncogene 2010; 29: 4671–4681.
Carro MS, Spiga FM, Quarto M, Di Ninni V, Volorio S, Alcalay M et al. DEK Expression is controlled by E2F and deregulated in diverse tumor types. Cell Cycle 2006; 5: 1202–1207.
Privette Vinnedge LM, Ho SM, Wikenheiser-Brokamp KA, Wells SI . The DEK oncogene is a target of steroid hormone receptor signaling in breast cancer. PLoS ONE 2012; 7: e46985.
Wise-Draper TM, Allen HV, Thobe MN, Jones EE, Habash KB, Munger K et al. The human DEK proto-oncogene is a senescence inhibitor and an upregulated target of high-risk human papillomavirus E7. J Virol 2005; 79: 14309–14317.
Evans AJ, Gallie BL, Jewett MA, Pond GR, Vandezande K, Underwood J et al. Defining a 0.5-mb region of genomic gain on chromosome 6p22 in bladder cancer by quantitative-multiplex polymerase chain reaction. Am J Pathol 2004; 164: 285–293.
Adams AK, Hallenbeck GE, Casper KA, Patil YJ, Wilson KM, Kimple RJ et al. DEK promotes HPV-positive and -negative head and neck cancer cell proliferation. Oncogene (e-pub ahead of print 10 March 2014; doi:10.1038/onc.2014.15).
Sanden C, Ageberg M, Petersson J, Lennartsson A, Gullberg U . Forced expression of the DEK-NUP214 fusion protein promotes proliferation dependent on upregulation of mTOR. BMC Cancer 2013; 13: 440.
McClaine RJ, Marshall AM, Wagh PK, Waltz SE . Ron receptor tyrosine kinase activation confers resistance to tamoxifen in breast cancer cell lines. Neoplasia 2010; 12: 650–658.
Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D et al. Purification and unique properties of mammary epithelial stem cells. Nature 2006; 439: 993–997.
Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML et al. Generation of a functional mammary gland from a single stem cell. Nature 2006; 439: 84–88.
Ma J, Lanza DG, Guest I, Uk-Lim C, Glinskii A, Glinsky G et al. Characterization of mammary cancer stem cells in the MMTV-PyMT mouse model. Tumour Biol 2012; 33: 1983–1996.
Wu B, Crampton SP, Hughes CC . Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity 2007; 26: 227–239.
Lane TF, Leder P . Wnt-10b directs hypermorphic development and transformation in mammary glands of male and female mice. Oncogene 1997; 15: 2133–2144.
Miyoshi K, Rosner A, Nozawa M, Byrd C, Morgan F, Landesman-Bollag E et al. Activation of different Wnt/beta-catenin signaling components in mammary epithelium induces transdifferentiation and the formation of pilar tumors. Oncogene 2002; 21: 5548–5556.
Debnath J, Muthuswamy SK, Brugge JS . Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 2003; 30: 256–268.
Habib SJ, Chen BC, Tsai FC, Anastassiadis K, Meyer T, Betzig E et al. A localized Wnt signal orients asymmetric stem cell division in vitro. Science 2013; 339: 1445–1448.
Karner C, Wharton KA, Carroll TJ . Apical–basal polarity, Wnt signaling and vertebrate organogenesis. Semin Cell Dev Biol 2006; 17: 214–222.
Gao B . Wnt regulation of planar cell polarity (PCP). Curr Top Dev Biol 2012; 101: 263–295.
Wise-Draper TM, Allen HV, Jones EE, Habash KB, Matsuo H, Wells SI . Apoptosis inhibition by the human DEK oncoprotein involves interference with p53 functions. Mol Cell Biol 2006; 26: 7506–7519.
Moll UM, Slade N . P63 and p73: roles in development and tumor formation. Mol Cancer Res 2004; 2: 371–386.
Wise-Draper TM, Morreale RJ, Morris TA, Mintz-Cole RA, Hoskins EE, Balsitis SJ et al. DEK proto-oncogene expression interferes with the normal epithelial differentiation program. Am J Pathol 2009; 174: 71–81.
Wend P, Runke S, Wend K, Anchondo B, Yesayan M, Jardon M et al. WNT10B/beta-catenin signalling induces HMGA2 and proliferation in metastatic triple-negative breast cancer. EMBO Mol Med 2013; 5: 264–279.
Veltmaat JM, Van Veelen W, Thiery JP, Bellusci S . Identification of the mammary line in mouse by Wnt10b expression. Dev Dyn 2004; 229: 349–356.
Miranda-Carboni GA, Krum SA, Yee K, Nava M, Deng QE, Pervin S et al. A functional link between Wnt signaling and SKP2-independent p27 turnover in mammary tumors. Genes Dev 2008; 22: 3121–3134.
Benhaj K, Akcali KC, Ozturk M . Redundant expression of canonical Wnt ligands in human breast cancer cell lines. Oncol Rep 2006; 15: 701–707.
Meier-Abt F, Milani E, Roloff T, Brinkhaus H, Duss S, Meyer DS et al. Parity induces differentiation and reduces Wnt/Notch signaling ratio and proliferation potential of basal stem/progenitor cells isolated from mouse mammary epithelium. Breast Cancer Res 2013; 15: R36.
Robinson GW, Hennighausen L, Johnson PF . Side-branching in the mammary gland: the progesterone–Wnt connection. Genes Dev 2000; 14: 889–894.
Shimizu H, Julius MA, Giarre M, Zheng Z, Brown AM, Kitajewski J . Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ 1997; 8: 1349–1358.
Huguet EL, McMahon JA, McMahon AP, Bicknell R, Harris AL . Differential expression of human Wnt genes 2, 3, 4, and 7B in human breast cell lines and normal and disease states of human breast tissue. Cancer Res 1994; 54: 2615–2621.
Kuorelahti A, Rulli S, Huhtaniemi I, Poutanen M . Human chorionic gonadotropin (hCG) upregulates wnt5b and wnt7b in the mammary gland, and hCGbeta transgenic female mice present with mammary gland tumors exhibiting characteristics of the Wnt/beta-catenin pathway activation. Endocrinology 2007; 148: 3694–3703.
Yin YJ, Katz V, Salah Z, Maoz M, Cohen I, Uziely B et al. Mammary gland tissue targeted overexpression of human protease-activated receptor 1 reveals a novel link to beta-catenin stabilization. Cancer Res 2006; 66: 5224–5233.
Rajagopal J, Carroll TJ, Guseh JS, Bores SA, Blank LJ, Anderson WJ et al. Wnt7b stimulates embryonic lung growth by coordinately increasing the replication of epithelium and mesenchyme. Development 2008; 135: 1625–1634.
Howe LR, Brown AM . Wnt signaling and breast cancer. Cancer Biol Ther 2004; 3: 36–41.
Florian MC, Nattamai KJ, Dorr K, Marka G, Uberle B, Vas V et al. A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature 2013; 503: 392–396.
Schlessinger K, Hall A, Tolwinski N . Wnt signaling pathways meet Rho GTPases. Genes Dev 2009; 23: 265–277.
Wang J, Sun L, Yang M, Luo W, Gao Y, Liu Z et al. DEK depletion negatively regulates Rho/ROCK/MLC pathway in non-small cell lung cancer. J Histochem Cytochem 2013; 61: 510–521.
Krol M, Polanska J, Pawlowski KM, Turowski P, Skierski J, Majewska A et al. Transcriptomic signature of cell lines isolated from canine mammary adenocarcinoma metastases to lungs. J Appl Genet 2010; 51: 37–50.
Wang MH, Zhang R, Zhou YQ, Yao HP . Pathogenesis of RON receptor tyrosine kinase in cancer cells: activation mechanism, functional crosstalk, and signaling addiction. J Biomed Res 2013; 27: 345–356.
Schneider CA, Rasband WS, Eliceiri KW . NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9: 671–675.
Euhus DM, Hudd C, LaRegina MC, Johnson FE . Tumor measurement in the nude mouse. J Surg Oncol 1986; 31: 229–234.
Tomayko MM, Reynolds CP . Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol 1989; 24: 148–154.
Peace BE, Toney-Earley K, Collins MH, Waltz SE . Ron receptor signaling augments mammary tumor formation and metastasis in a murine model of breast cancer. Cancer Res 2005; 65: 1285–1293.
Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat 2010; 123: 725–731.
Acknowledgements
We acknowledge James Lessard and Gerard Grosveld for reagents, Kathryn Wikenheiser-Brokamp for technical assistance and the Research Flow Cytometry Core at Cincinnati Children’s, supported by NIH AR-47363. LMPV is supported by the Ride Cincinnati Foundation for Breast Cancer Research in honor of Marlene Harris and Public Health Service Grants F32CA139931, T32HL091805 and K12HD051953. Funding also was provided by Public Health Services Grants R01CA116316 (SIW), R01HL875109 (JAC), T32ES007250 (AKA), T32CA117846-07 (PKW) and T32CA11784 (SEW and NMB), Department of Defense Grant W81XWH-12-1-0194 (SIW and SEW) and the Veteran’s Administration Grant BX000803 (SEW). JS-W is supported by the Government of the Spanish Junta de Andalucia.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Privette Vinnedge, L., Benight, N., Wagh, P. et al. The DEK oncogene promotes cellular proliferation through paracrine Wnt signaling in Ron receptor-positive breast cancers. Oncogene 34, 2325–2336 (2015). https://doi.org/10.1038/onc.2014.173
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.173
This article is cited by
-
A network map of macrophage-stimulating protein (MSP) signaling
Journal of Cell Communication and Signaling (2023)
-
Epigenetic regulation of breast cancer metastasis
Cancer and Metastasis Reviews (2023)
-
Exosomal DEK removes chemoradiotherapy resistance by triggering quiescence exit of breast cancer stem cells
Oncogene (2022)
-
A three layered histone epigenetics in breast cancer metastasis
Cell & Bioscience (2020)
-
MST1R (RON) expression is a novel prognostic biomarker for metastatic progression in breast cancer patients
Breast Cancer Research and Treatment (2020)