Abstract
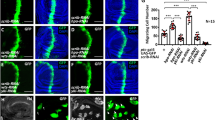
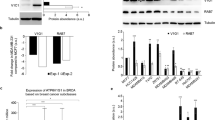
Tumor cell migration has a fundamental role in early steps of metastasis, the fatal hallmark of cancer. In the present study, we investigated the effects of the tyrosine phosphatase, SRC-homology 2 domain-containing phosphatase 2 (SHP2), on cell migration in metastatic triple-negative breast cancer (TNBC), an aggressive disease associated with a poor prognosis for which a targeted therapy is not yet available. Using mouse models and multiphoton intravital imaging, we have identified a crucial effect of SHP2 on TNBC cell motility in vivo. Further, analysis of TNBC cells revealed that SHP2 also influences cell migration, chemotaxis and invasion in vitro. Unbiased phosphoproteomics and biochemical analysis showed that SHP2 activates several SRC-family kinases and downstream targets, most of which are inducers of migration and invasion. In particular, direct interaction between SHP2 and c-SRC was revealed by a fluorescence resonance energy transfer assay. These results suggest that SHP2 is a crucial factor during early steps of TNBC migration to distant organs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Perou CM Molecular stratification of triple-negative breast cancers. Oncologist 2010; 15: 39–48.
Aceto N, Sausgruber N, Brinkhaus H, Gaidatzis D, Martiny-Baron G, Mazzarol G et al. Tyrosine phosphatase SHP2 promotes breast cancer progression and maintains tumor-initiating cells via activation of key transcription factors and a positive feedback signaling loop. Nat Med 2012; 18: 529–537.
Wang FM, Liu HQ, Liu SR, Tang SP, Yang L, Feng GS . SHP-2 promoting migration and metastasis of MCF-7 with loss of E-cadherin, dephosphorylation of FAK and secretion of MMP-9 induced by IL-1beta in vivo and in vitro. Breast Cancer Res Treat 2005; 89: 5–14.
Patsialou A, Wang Y, Lin J, Whitney K, Goswami S, Kenny PA et al. Selective gene-expression profiling of migratory tumor cells in vivo predicts clinical outcome in breast cancer patients. Breast Cancer Res 2012; 14: R139.
Bonapace L, Wyckoff J, Oertner T, Van Rheenen J, Junt T, Bentires-Alj M If you don't look, you won't see: intravital multiphoton imaging of primary and metastatic breast cancer. J Mammary Gland Biol Neoplasia 2012; 17: 125–129.
Zomer A, Beerling E, Vlug EJ, van Rheenen J Real-time intravital imaging of cancer models. Clin Transl Oncol 2011; 13: 848–854.
Liu H, Patel MR, Prescher JA, Patsialou A, Qian D, Lin J et al. Cancer stem cells from human breast tumors are involved in spontaneous metastases in orthotopic mouse models. Proc Natl Acad Sci USA 2010; 107: 18115–18120.
Pawitan Y, Bjohle J, Amler L, Borg AL, Egyhazi S, Hall P et al. Gene expression profiling spares early breast cancer patients from adjuvant therapy: derived and validated in two population-based cohorts. Breast Cancer Res 2005; 7: R953–R964.
Yamaguchi H, Wyckoff J, Condeelis J Cell migration in tumors. Curr Opin Cell Biol 2005; 17: 559–564.
Zhang SQ, Yang W, Kontaridis MI, Bivona TG, Wen G, Araki T et al. Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol Cell 2004; 13: 341–355.
Ishizawar R, Parsons SJ c-Src and cooperating partners in human cancer. Cancer Cell 2004; 6: 209–214.
Ren Y, Meng S, Mei L, Zhao ZJ, Jove R, Wu J Roles of Gab1 and SHP2 in paxillin tyrosine dephosphorylation and Src activation in response to epidermal growth factor. J Biol Chem 2004; 279: 8497–8505.
Peng ZY, Cartwright CA Regulation of the Src tyrosine kinase and Syp tyrosine phosphatase by their cellular association. Oncogene 1995; 11: 1955–1962.
Sachdev S, Bu Y, Gelman IH Paxillin-Y118 phosphorylation contributes to the control of Src-induced anchorage-independent growth by FAK and adhesion. BMC Cancer 2009; 9: 12.
Roof RW, Haskell MD, Dukes BD, Sherman N, Kinter M, Parsons SJ Phosphotyrosine (p-Tyr)-dependent and -independent mechanisms of p190 RhoGAP-p120 RasGAP interaction: Tyr 1105 of p190, a substrate for c-Src, is the sole p-Tyr mediator of complex formation. Mol Cell Biol 1998; 18: 7052–7063.
Sanders MA, Basson MD p130cas but not paxillin is essential for Caco-2 intestinal epithelial cell spreading and migration on collagen IV. J Biol Chem 2005; 280: 23516–23522.
Iwasaki T, Nakata A, Mukai M, Shinkai K, Yano H, Sabe H et al. Involvement of phosphorylation of Tyr-31 and Tyr-118 of paxillin in MM1 cancer cell migration. Int J Cancer 2002; 97: 330–335.
Nakamura K, Yano H, Uchida H, Hashimoto S, Schaefer E, Sabe H . Tyrosine phosphorylation of paxillin alpha is involved in temporospatial regulation of paxillin-containing focal adhesion formation and F-actin organization in motile cells. J Biol Chem 2000; 275: 27155–27164.
Klinghoffer RA, Sachsenmaier C, Cooper JA, Soriano P Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J 1999; 18: 2459–2471.
Schober M, Raghavan S, Nikolova M, Polak L, Pasolli HA, Beggs HE et al. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J Cell Biol 2007; 176: 667–680.
Fincham VJ, Frame MC The catalytic activity of Src is dispensable for translocation to focal adhesions but controls the turnover of these structures during cell motility. EMBO J 1998; 17: 81–92.
Yu DH, Qu CK, Henegariu O, Lu X, Feng GS Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J Biol Chem 1998; 273: 21125–21131.
Manes S, Mira E, Gomez-Mouton C, Zhao ZJ, Lacalle RA, Martinez AC Concerted activity of tyrosine phosphatase SHP-2 and focal adhesion kinase in regulation of cell motility. Mol Cell Biol 1999; 19: 3125–3135.
Hartman ZR, Schaller MD, Agazie YM The tyrosine phosphatase SHP2 regulates focal adhesion kinase to promote EGF-induced lamellipodia persistence and cell migration. Mol Cancer Res 2013; 11: 651–664.
Minn AJ, Gupta GP, Padua D, Bos P, Nguyen DX, Nuyten D et al. Lung metastasis genes couple breast tumor size and metastatic spread. Proc Natl Acad Sci USA 2007; 104: 6740–6745.
Wang W, Wyckoff JB, Frohlich VC, Oleynikov Y, Huttelmaier S, Zavadil J et al. Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res 2002; 62: 6278–6288.
Britschgi A, Andraos R, Brinkhaus H, Klebba I, Romanet V, Muller U et al. JAK2/STAT5 inhibition circumvents resistance to PI3K/mTOR blockade: a rationale for cotargeting these pathways in metastatic breast cancer. Cancer Cell 2012; 22: 796–811.
Meijering E, Dzyubachyk O, Smal I Methods for cell and particle tracking. Methods Enzymol 2012; 504: 183–200.
Dey JH, Bianchi F, Voshol J, Bonenfant D, Oakeley EJ, Hynes NE Targeting fibroblast growth factor receptors blocks PI3K/AKT signaling, induces apoptosis, and impairs mammary tumor outgrowth and metastasis. Cancer Res 2010; 70: 4151–4162.
Acknowledgements
We thank C Kuperwasser for the SUM159 cells and S Gambhir for the pFU-Luc2-eGFP construct, L Bonapace for technical assistance with the intravital microscope, L Gelman for help with the FRET experiments, S Bourke and M Kirschmann for help with image acquisition and analysis, and I Klebba and H Brinkhaus for technical assistance. M-MC is supported by a FP7 Marie-Curie Fellowship and NA is a fellow of the Swiss National Science Foundation and the Human Frontiers Science Program. Research in the laboratory of MB-A is supported by the Novartis Research Foundation, the European Research Council (ERC starting grant 243211-PTPsBDC), the Swiss Cancer League and the Krebsliga Beider Basel.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Sausgruber, N., Coissieux, MM., Britschgi, A. et al. Tyrosine phosphatase SHP2 increases cell motility in triple-negative breast cancer through the activation of SRC-family kinases. Oncogene 34, 2272–2278 (2015). https://doi.org/10.1038/onc.2014.170
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.170
This article is cited by
-
Cathepsin K inhibition induces Raptor destabilization and mitochondrial dysfunction via Syk/SHP2/Src/OTUB1 axis-mediated signaling
Cell Death & Disease (2023)
-
PI3K inhibition circumvents resistance to SHP2 blockade in metastatic triple-negative breast cancer
Journal of Mammary Gland Biology and Neoplasia (2023)
-
SRC kinase-mediated signaling pathways and targeted therapies in breast cancer
Breast Cancer Research (2022)
-
Lipidomic and Membrane Mechanical Signatures in Triple-Negative Breast Cancer: Scope for Membrane-Based Theranostics
Molecular and Cellular Biochemistry (2022)
-
Statistical assessment and visualization of synergies for large-scale sparse drug combination datasets
BMC Bioinformatics (2019)