Abstract
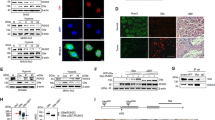
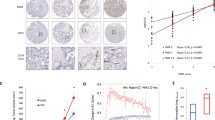
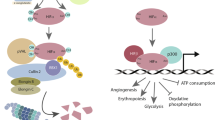
RUNX3 is silenced by histone modification and hypoxia-inducible factor (HIF)-1α is stabilized under hypoxia, but little is known of cross-talk between RUNX3 and HIF-1α under hypoxia. In the present study, the authors investigated the effect of RUNX3 on HIF-1α stability in gastric cancer cells. RUNX3 overexpression was found to downregulate HIF-1α stability under normoxic and hypoxic conditions. Furthermore, the activity of a luciferase reporter containing five copies of vascular endothelial growth factor (VEGF) promoter hypoxia-responsive element (5 × HRE) and the amount of secreted VEGF, were diminished in RUNX3-expressing but increased in RUNX3-knockdown cells. When expression of RUNX3 was recovered using epigenetic reagents the expressions of HIF-1α and VEGF were clearly suppressed under hypoxic conditions. RUNX3 also significantly attenuated the half-life of HIF-1α protein, and induced the cytosolic localization and ubiquitination of HIF-1α. In addition, RUNX3 directly interacted with the C-terminal activation domain of HIF-1α and prolyl hydroxylase (PHD) 2 and enhanced the interaction between HIF-1α and PHD2, which potentiated proline hydroxylation and promoted the degradation of HIF-1α. Furthermore, RUNX3 overexpression significantly inhibited hypoxia-induced angiogenesis in vitro and in vivo. Taken together, these results suggest that RUNX3 destabilizes HIF-1α protein by promoting the proline hydroxylation of HIF-1α through binding to HIF-1α/PHD2. RUNX3 appears to be a novel suppressor of HIF-1α and of hypoxia-mediated angiogenesis in gastric cancer cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Lee SH, Kim J, Kim WH, Lee YM . Hypoxic silencing of tumor suppressor RUNX3 by histone modification in gastric cancer cells. Oncogene 2009; 28: 184–194.
Semenza GL . Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med 2003; 54: 17–28.
Harris AL . Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer 2002; 2: 38–47.
Folkman J . What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 1990; 82: 4–6.
Cao Y, Liu Q . Therapeutic targets of multiple angiogenic factors for the treatment of cancer and metastasis. Adv Cancer Res 2007; 97: 203–224.
Salceda S, Caro J . Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem 1997; 272: 22642–22647.
Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol 2000; 2: 423–427.
Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ . Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J 2001; 20: 5197–5206.
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 2001; 292: 464–468.
Li QL, Ito K, Sakakura C, Fukamachi H, Inoue K, Chi XZ et al. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell 2002; 109: 113–124.
Chuang LS, Ito Y . RUNX3 is multifunctional in carcinogenesis of multiple solid tumors. Oncogene 2010; 29: 2605–2615.
Wei D, Gong W, Oh SC, Li Q, Kim WD, Wang L et al. Loss of RUNX3 expression significantly affects the clinical outcome of gastric cancer patients and its restoration causes drastic suppression of tumor growth and metastasis. Cancer Res 2005; 65: 4809–4816.
Lund AH, van Lohuizen M . RUNX: a trilogy of cancer genes. Cancer Cell 2002; 1: 213–215.
Ito Y . RUNX genes in development and cancer: regulation of viral gene expression and the discovery of RUNX family genes. Adv Cancer Res 2008; 99: 33–76.
Peng Z, Wei D, Wang L, Tang H, Zhang J, Le X et al. RUNX3 inhibits the expression of vascular endothelial growth factor and reduces the angiogenesis, growth, and metastasis of human gastric cancer. Clin Cancer Res 2006; 12: 6386–6394.
Jeong CH, Lee YM, Choi KS, Seong YR, Kim YJ, Im DS et al. Hypoxia-responsive element-mediated soluble Tie2 vector exhibits an anti-angiogenic activity in vitro under hypoxic condition. Int J Oncol 2005; 26: 211–216.
Takeda K, Ichiki T, Narabayashi E, Inanaga K, Miyazaki R, Hashimoto T et al. Inhibition of prolyl hydroxylase domain-containing protein suppressed lipopolysaccharide-induced TNF-alpha expression. Arterioscler Thromb Vasc Biol 2009; 29: 2132–2137.
Pore N, Jiang Z, Shu HK, Bernhard E, Kao GD, Maity A . Akt1 activation can augment hypoxia-inducible factor-1alpha expression by increasing protein translation through a mammalian target of rapamycin-independent pathway. Mol Cancer Res 2006; 4: 471–479.
Corish P, Tyler-Smith C . Attenuation of green fluorescent protein half-life in mammalian cells. Protein Eng 1999; 12: 1035–1040.
Bae MK, Jeong JW, Kim SH, Kim SY, Kang HJ, Kim DM et al. Tid-1 interacts with the von Hippel-Lindau protein and modulates angiogenesis by destabilization of HIF-1alpha. Cancer Res 2005; 65: 2520–2525.
Hagen T, Taylor CT, Lam F, Moncada S . Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science 2003; 302: 1975–1978.
Semenza GL . HIF-1: using two hands to flip the angiogenic switch. Cancer Metastasis Rev 2000; 19: 59–65.
Kaelin WG, Ratcliffe PJ . Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 2008; 30: 393–402.
An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM . Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature 1998; 392: 405–408.
Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM et al. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res 2000; 60: 1541–1545.
Jeong JW, Bae MK, Ahn MY, Kim SH, Sohn TK, Bae MH et al. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell 2002; 111: 709–720.
Lee MN, Lee SN, Kim SH, Kim B, Jung BK, Seo JH et al. Roles of arrest-defective protein 1(225) and hypoxia-inducible factor 1alpha in tumor growth and metastasis. J Natl Cancer Inst 2010; 102: 426–442.
Coulon C, Georgiadou M, Roncal C, De Bock K, Langenberg T, Carmeliet P . From vessel sprouting to normalization: role of the prolyl hydroxylase domain protein/hypoxia-inducible factor oxygen-sensing machinery. Arterioscler Thromb Vasc Biol 2010; 30: 2331–2336.
Semenza GL . Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003; 3: 721–732.
Fujita N, Markova D, Anderson DG, Chiba K, Toyama Y, Shapiro IM et al. Expression of prolyl hydroxylases (PHDs) is selectively controlled by HIF-1 and HIF-2 proteins in nucleus pulposus cells of the intervertebral disc: distinct roles of PHD2 and PHD3 proteins in controlling HIF-1alpha activity in hypoxia. J Biol Chem 2012; 287: 16975–16986.
Chan DA, Sutphin PD, Yen SE, Giaccia AJ . Coordinate regulation of the oxygen-dependent degradation domains of hypoxia-inducible factor 1 alpha. Mol Cell Biol 2005; 25: 6415–6426.
Stiehl DP, Wirthner R, Koditz J, Spielmann P, Camenisch G, Wenger RH . Increased prolyl 4-hydroxylase domain proteins compensate for decreased oxygen levels. Evidence for an autoregulatory oxygen-sensing system. J Biol Chem 2006; 281: 23482–23491.
Poon E, Harris AL, Ashcroft M . Targeting the hypoxia-inducible factor (HIF) pathway in cancer. Expert Rev Mol Med 2009; 11: e26.
Zhang Y, Li M, Yao Q, Chen C . Recent advances in tumor hypoxia: tumor progression, molecular mechanisms, and therapeutic implications. Med Sci Monit 2007; 13: RA175–RA180.
Guo WH, Weng LQ, Ito K, Chen LF, Nakanishi H, Tatematsu M et al. Inhibition of growth of mouse gastric cancer cells by Runx3, a novel tumor suppressor. Oncogene 2002; 21: 8351–8355.
Ito K, Liu Q, Salto-Tellez M, Yano T, Tada K, Ida H et al. RUNX3, a novel tumor suppressor, is frequently inactivated in gastric cancer by protein mislocalization. Cancer Res 2005; 65: 7743–7750.
Yano T, Ito K, Fukamachi H, Chi XZ, Wee HJ, Inoue K et al. The RUNX3 tumor suppressor upregulates Bim in gastric epithelial cells undergoing transforming growth factor beta-induced apoptosis. Mol Cell Biol 2006; 26: 4474–4488.
Lau QC, Raja E, Salto-Tellez M, Liu Q, Ito K, Inoue M et al. RUNX3 is frequently inactivated by dual mechanisms of protein mislocalization and promoter hypermethylation in breast cancer. Cancer Res 2006; 66: 6512–6520.
Kim HR, Oh BC, Choi JK, Bae SC . Pim-1 kinase phosphorylates and stabilizes RUNX3 and alters its subcellular localization. J Cell Biochem 2008; 105: 1048–1058.
Goh YM, Cinghu S, Hong ET, Lee YS, Kim JH, Jang JW et al. Src kinase phosphorylates RUNX3 at tyrosine residues and localizes the protein in the cytoplasm. J Biol Chem 2010; 285: 10122–10129.
Kim JH, Choi JK, Cinghu S, Jang JW, Lee YS, Li YH et al. Jab1/CSN5 induces the cytoplasmic localization and degradation of RUNX3. J Cell Biochem 2009; 107: 557–565.
Chang CC, Lin BR, Chen ST, Hsieh TH, Li YJ, Kuo MY . HDAC2 promotes cell migration/invasion abilities through HIF-1alpha stabilization in human oral squamous cell carcinoma. J Oral Pathol Med 2011; 40: 567–575.
Kim MS, Kwon HJ, Lee YM, Baek JH, Jang JE, Lee SW et al. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med 2001; 7: 437–443.
Ellis L, Hammers H, Pili R . Targeting tumor angiogenesis with histone deacetylase inhibitors. Cancer Lett 2009; 280: 145–153.
Nakase Y, Sakakura C, Miyagawa K, Kin S, Fukuda K, Yanagisawa A et al. Frequent loss of RUNX3 gene expression in remnant stomach cancer and adjacent mucosa with special reference to topography. Br J Cancer 2005; 92: 562–569.
Isobe T, Aoyagi K, Koufuji K, Shirouzu K, Kawahara A, Taira T et al. Clinicopathological significance of hypoxia-inducible factor-1 alpha (HIF-1α) expression in gastric cancer. Int J Clin Oncol 2012, (e-pub ahead of printer 18 February 2012).
Acknowledgements
This work was supported by a National Research Foundation grant from the Basic Research Lab program (2012-028835) and from Mid-career Researcher Program (2010-0026741) funded by the Korean Ministry of Education, Science Technology (MEST).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Lee, S., Bae, S., Kim, K. et al. RUNX3 inhibits hypoxia-inducible factor-1α protein stability by interacting with prolyl hydroxylases in gastric cancer cells. Oncogene 33, 1458–1467 (2014). https://doi.org/10.1038/onc.2013.76
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.76
Keywords
This article is cited by
-
Foxp1 suppresses cortical angiogenesis and attenuates HIF-1alpha signaling to promote neural progenitor cell maintenance
EMBO Reports (2024)
-
MicroRNA-495: a therapeutic and diagnostic tumor marker
Journal of Molecular Histology (2023)
-
Relation of the methylation state of RUNX3 and p16 gene promoters with hepatocellular carcinoma in Egyptian patients
Egyptian Journal of Medical Human Genetics (2022)
-
Myeloid neoplasms and clonal hematopoiesis from the RUNX1 perspective
Leukemia (2022)
-
The multifaceted role of EGLN family prolyl hydroxylases in cancer: going beyond HIF regulation
Oncogene (2022)