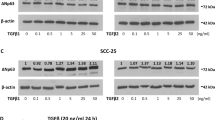
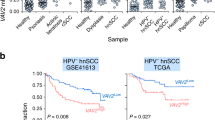
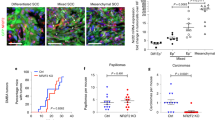
Abstract
Most of the squamous cell carcinomas (SCCs) of the skin and head and neck contain p53 mutations. The presence of p53 mutations in premalignant lesions suggests that they represent early events during tumor progression and additional alterations may be required for SCC development. Here we show that codeletion of the p53 and αv integrin genes in mouse stratified epithelia induced SCCs in 100% of the mice, more frequently and with much shorter latency than deletion of either gene alone. The SCCs that lacked p53 and αv in the epithelial tumor cells exhibited high Akt activity, lacked multiple types of infiltrating immune cells, contained a defective vasculature and grew slower than tumors that expressed p53 or αv. These results reveal that loss of αv in epithelial cells that lack p53 promotes SCC development, but also prevents remodeling of the tumor microenvironment and delays tumor growth. We observed that Akt inactivation in SCC cells that lack p53 and αv promoted anoikis. Thus, tumors may arise in these mice as a result of the increased cell survival induced by Akt activation triggered by loss of αv and p53, and by the defective recruitment of immune cells to these tumors, which may allow immune evasion. However, the defective vasculature and lack of a supportive stroma create a restrictive microenvironment in these SCCs that slows their growth. These mechanisms may underlie the rapid onset and slow growth of SCCs that lack p53 and αv.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Brantsch KD, Meisner C, Schonfisch B, Trilling B, Wehner-Caroli J, Rocken M et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol 2008; 9: 713–720.
Lindelof B, Sigurgeirsson B, Gabel H, Stern RS . Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol 2000; 143: 513–519.
Euvrard S, Kanitakis J, Claudy A . Skin cancers after organ transplantation. N Engl J Med 2003; 348: 1681–1691.
Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP et al. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci USA 1991; 88: 10124–10128.
Bolshakov S, Walker CM, Strom SS, Selvan MS, Clayman GL, El Naggar A et al. P53 mutations in human aggressive and nonaggressive basal and squamous cell carcinomas. Clin Cancer Res 2003; 9: 228–234.
Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 2011; 333: 1154–1157.
Campbell C, Quinn AG, Ro YS, Angus B, Rees JL . P53 mutations are common and early events that precede tumor invasion in squamous cell neoplasia of the skin. J Invest Dermatol 1993; 100: 746–748.
Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J et al. Sunburn and p53 in the onset of skin cancer. Nature 1994; 372: 773–776.
Nakazawa H, English D, Randell PL, Nakazawa K, Martel N, Armstrong B et al. UV and skin cancer: specific p53 gene mutation in normal skin as a biologically relevant exposure measurement. Proc Natl Acad Sci USA 1994; 91: 360–364.
Jonason AS, Kunala S, Price GJ, Restifo RJ, Spinelli HM, Persing JA et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci USA 1996; 93: 14025–14029.
Jonkers J, Meuwissen R, van der GH, Peterse H, van d V, Berns A . Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet 2001; 29: 418–425.
Caulin C, Nguyen T, Lang GA, Goepfert TM, Brinkley BR, Cai WW et al. An inducible mouse model for skin cancer reveals distinct roles for gain- and loss-of-function p53 mutations. J Clin Invest 2007; 117: 1893–1901.
Martinez-Cruz AB, Santos M, Lara MF, Segrelles C, Ruiz S, Moral M et al. Spontaneous squamous cell carcinoma induced by the somatic inactivation of retinoblastoma and Trp53 tumor suppressors. Cancer Res 2008; 68: 683–692.
Watt FM . Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J 2002; 21: 3919–3926.
Hynes RO . Integrins: bidirectional, allosteric signaling machines. Cell 2002; 110: 673–687.
De LM, Tamura RN, Kajiji S, Bondanza S, Rossino P, Cancedda R et al. Polarized integrin mediates human keratinocyte adhesion to basal lamina. Proc Natl Acad Sci USA 1990; 87: 6888–6892.
Peltonen J, Larjava H, Jaakkola S, Gralnick H, Akiyama SK, Yamada SS et al. Localization of integrin receptors for fibronectin, collagen, and laminin in human skin. Variable expression in basal and squamous cell carcinomas. J Clin Invest 1989; 84: 1916–1923.
Hertle MD, Kubler MD, Leigh IM, Watt FM . Aberrant integrin expression during epidermal wound healing and in psoriatic epidermis. J Clin Invest 1992; 89: 1892–1901.
Haapasalmi K, Zhang K, Tonnesen M, Olerud J, Sheppard D, Salo T et al. Keratinocytes in human wounds express alpha v beta 6 integrin. J Invest Dermatol 1996; 106: 42–48.
Janes SM, Watt FM . New roles for integrins in squamous-cell carcinoma. Nat Rev Cancer 2006; 6: 175–183.
Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF et al. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci 1995; 108 (Part 6): 2241–2251.
Jones J, Watt FM, Speight PM . Changes in the expression of alpha v integrins in oral squamous cell carcinomas. J Oral Pathol Med 1997; 26: 63–68.
Hsu A, Esmaeli B, Hayek B, Hossain MG, Shinder R, Lazar AJ et al. Analysis of alphav integrin protein expression in human eyelid and periorbital squamous cell carcinomas. J Cutan Pathol 2011; 38: 570–575.
McCarty JH, Barry M, Crowley D, Bronson RT, Lacy-Hulbert A, Hynes RO . Genetic ablation of alphav integrins in epithelial cells of the eyelid skin and conjunctiva leads to squamous cell carcinoma. Am J Pathol 2008; 172: 1740–1747.
McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D et al. Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development 2005; 132: 165–176.
Caulin C, Nguyen T, Longley MA, Zhou Z, Wang XJ, Roop DR . Inducible activation of oncogenic K-ras results in tumor formation in the oral cavity. Cancer Res 2004; 64: 5054–5058.
Nappi O, Pettinato G, Wick MR . Adenoid (acantholytic) squamous cell carcinoma of the skin. J Cutan Pathol 1989; 16: 114–121.
Streuli CH, Akhtar N . Signal co-operation between integrins and other receptor systems. Biochem J 2009; 418: 491–506.
Janes SM, Watt FM . Switch from alphavbeta5 to alphavbeta6 integrin expression protects squamous cell carcinomas from anoikis. J Cell Biol 2004; 166: 419–431.
Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999; 96: 319–328.
Jin H, Su J, Garmy-Susini B, Kleeman J, Varner J . Integrin alpha4beta1 promotes monocyte trafficking and angiogenesis in tumors. Cancer Res 2006; 66: 2146–2152.
Torchia EC, Caulin C, Acin S, Terzian T, Kubick BJ, Box NF et al. Myc, aurora kinase A, and mutant p53(R172H) co-operate in a mouse model of metastatic skin carcinoma. Oncogene 2011; 31: 2680–2690.
Spike BT, Wahl GM . P53, stem cells, and reprogramming: tumor suppression beyond guarding the genome. Genes Cancer 2011; 2: 404–419.
Aladjem MI, Spike BT, Rodewald LW, Hope TJ, Klemm M, Jaenisch R et al. ES cells do not activate p53-dependent stress responses and undergo p53-independent apoptosis in response to DNA damage. Curr Biol 1998; 8: 145–155.
Strömblad S, Fotedar A, Brickner H, Theesfeld C, Aguilar de Diaz E, Friedlander M et al. Loss of p53 compensates for alpha v-integrin function in retinal neovascularization. J Biol Chem 2002; 277: 13371–13374.
Bao W, Stromblad S . Integrin alphav-mediated inactivation of p53 controls a MEK1-dependent melanoma cell survival pathway in three-dimensional collagen. J Cell Biol 2004; 167: 745–756.
Jones J, Sugiyama M, Speight PM, Watt FM . Restoration of alpha v beta 5 integrin expression in neoplastic keratinocytes results in increased capacity for terminal differentiation and suppression of anchorage-independent growth. Oncogene 1996; 12: 119–126.
Hanahan D, Coussens LM . Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21: 309–322.
Mantovani A, Allavena P, Sica A, Balkwill F . Cancer-related inflammation. Nature 2008; 454: 436–444.
Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Bronson RT et al. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci USA 2007; 104: 15823–15828.
Acin S, Li Z, Mejia O, Roop DR, El-Naggar AK, Caulin C . Gain-of-function mutant p53 but not p53 deletion promotes head and neck cancer progression in response to oncogenic K-ras. J Pathol 2011; 225: 479–489.
Acknowledgements
We thank Dennis Roop for the K14.CrePR1 mice, Anton Berns for the floxed p53 mice and Sarah Bronson and Dawn Chalaire for editorial assistance. This study was supported by the National Institutes of Health through Grant DE015344 (C Caulin) and a Pilot Project grant from the American Cancer Society Institutional Research Grant Program (JH McCarty). Veterinary services and core facilities at The University of Texas MD Anderson Cancer Center were supported in part by the National Institutes of Health through Cancer Center Support Grant CA16672.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Savar, A., Acin, S., Gonzalez, C. et al. Loss of epithelial p53 and αv integrin cooperate through Akt to induce squamous cell carcinoma yet prevent remodeling of the tumor microenvironment. Oncogene 34, 516–524 (2015). https://doi.org/10.1038/onc.2013.585
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.585
Keywords
This article is cited by
-
Differential responses to immune checkpoint inhibitor dictated by pre-existing differential immune profiles in squamous cell carcinomas caused by same initial oncogenic drivers
Journal of Experimental & Clinical Cancer Research (2022)
-
Inhibition of skin carcinogenesis by suppression of NF-κB dependent ITGAV and TIMP-1 expression in IL-32γ overexpressed condition
Journal of Experimental & Clinical Cancer Research (2018)
-
Targeting to the non-genomic activity of retinoic acid receptor-gamma by acacetin in hepatocellular carcinoma
Scientific Reports (2017)