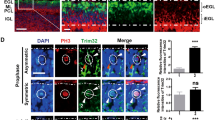
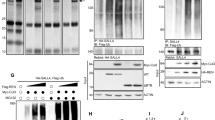
Abstract
Apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) is silenced by promoter methylation in many types of tumors, yet ASC’s role in most cancers remains unknown. Here, we show that ASC is highly expressed in a model of medulloblastoma, the most common malignant pediatric brain cancer; ASC is also expressed in human medulloblastomas. Importantly, while ASC deficiency did not affect normal cerebellar development, ASC knockout mice on the Smoothened (ND2:SmoA1) transgenic model of medulloblastoma exhibited a profound reduction in medulloblastoma incidence and a delayed tumor onset. A similar decrease in tumorigenesis with ASC deficiency was also seen in the hGFAP-Cre:SmoM2 mouse model of medulloblastoma. Interestingly, hyperproliferation of the external granule layer (EGL) was comparable at P20 in both wild-type and ASC-deficient SmoA1 mice. However, while the apoptosis and differentiation markers remained unchanged at this age, proliferation makers were decreased, and the EGL was reduced in thickness and area by P60. This reduction in proliferation with ASC deficiency was also seen in isolated SmoA1 cerebellar granule precursor cells in vitro, indicating that the effect of ASC deletion on proliferation was cell autonomous. Interestingly, ASC-deficient SmoA1 cerebella exhibited disrupted expression of genes in the transforming growth factor-β pathway and increased level of nuclear Smad3. Taken together, these results demonstrate an unexpected role for ASC in Sonic hedgehog-driven medulloblastoma tumorigenesis, thus identifying ASC as a promising novel target for antitumor therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Hatten M, Roussel M . Development and cancer of the cerebellum. Trends Neurosci 2011; 34: 134–142.
Hatten ME, Heintz N . Mechanisms of neural patterning and specification in the developing cerebellum. Annu Rev Neurosci 1995; 18: 385–408.
Polkinghorn WR, Tarbell NJ . Medulloblastoma: tumorigenesis, current clinical paradigm, and efforts to improve risk stratification. Nat Clin Pract Oncol 2007; 4: 295–304.
Yang ZJ, Ellis T, Markant SL, Read TA, Kessler JD, Bourboulas M et al. Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells. Cancer Cell 2008; 14: 135–145.
Hallahan AR, Pritchard JI, Hansen S, Benson M, Stoeck J, Hatton BA et al. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res 2004; 64: 7794–7800.
Stone AR, Bobo W, Brat DJ, Devi NS, Van Meir EG, Vertino PM . Aberrant methylation and down-regulation of TMS1/ASC in human glioblastoma. Am J Pathol 2004; 165: 1151–1161.
Alaminos M, Davalos V, Cheung NK, Gerald WL, Esteller M . Clustering of gene hypermethylation associated with clinical risk groups in neuroblastoma. J Natl Cancer Inst 2004; 96: 1208–1219.
Conway KE, McConnell BB, Bowring CE, Donald CD, Warren ST, Vertino PM . TMS1 a novel proapoptotic caspase recruitment domain protein, is a target of methylation-induced gene silencing in human breast cancers. Cancer Res 2000; 60: 6236–6242.
Guan X, Sagara J, Yokoyama T, Koganehira Y, Oguchi M, Saida T et al. ASC/TMS1, a caspase-1 activating adaptor, is downregulated by aberrant methylation in human melanoma. Int J Cancer 2003; 107: 202–208.
Machida EO, Brock MV, Hooker CM, Nakayama J, Ishida A, Amano J et al. Hypermethylation of ASC/TMS1 is a sputum marker for late-stage lung cancer. Cancer Res 2006; 66: 6210–6218.
Ramachandran K, Miller H, Gordian E, Rocha-Lima C, Singal R . Methylation-mediated silencing of TMS1 in pancreatic cancer and its potential contribution to chemosensitivity. Anticancer Res 2010; 30: 3919–3925.
Parsons MJ, Vertino PM . Dual role of TMS1/ASC in death receptor signaling. Oncogene 2006; 25: 6948–6958.
Ohtsuka T, Ryu H, Minamishima YA, Macip S, Sagara J, Nakayama KI et al. ASC is a Bax adaptor and regulates the p53-Bax mitochondrial apoptosis pathway. Nat Cell Biol 2004; 6: 121–128.
Ohtsuka T, Liu XF, Koga Y, Kitajima Y, Nakafusa Y, Ha CW et al. Methylation-induced silencing of ASC and the effect of expressed ASC on p53-mediated chemosensitivity in colorectal cancer. Oncogene 2006; 25: 1807–1811.
Hong S, Hwang I, Lee YS, Park S, Lee WK, Fernandes-Alnemri T et al. Restoration of ASC expression sensitizes colorectal cancer cells to genotoxic stress-induced caspase-independent cell death. Cancer Lett 2013; 331: 183–191.
Parsons MJ, Patel P, Brat DJ, Colbert L, Vertino PM . Silencing of TMS1/ASC promotes resistance to anoikis in breast epithelial cells. Cancer Res 2009; 69: 1706–1711.
Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES . AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 2009; 458: 509–513.
Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G . The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 2009; 10: 241–247.
Davis BK, Wen H, Ting JP . The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 2011; 29: 707–735.
Allen IC, TeKippe EM, Woodford R-MT, Uronis JM, Holl EK, Rogers AB et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med 2010; 207: 1045–1056.
Zaki MH, Vogel P, Body-Malapel M, Lamkanfi M, Kanneganti T-D . IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J Immunol 2010; 185: 4912–4920.
Kool M, Koster J, Bunt J, Hasselt NE, Lakeman A, van Sluis P et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One 2008; 3: e3088.
Fattet S, Haberler C, Legoix P, Varlet P, Lellouch-Tubiana A, Lair S et al. Beta-catenin status in paediatric medulloblastomas: correlation of immunohistochemical expression with mutational status, genetic profiles, and clinical characteristics. J Pathol 2009; 218: 86–94.
Feierabend D, Walter J, Grube S, Herbold C, Beetz C, Kalff R et al. Methylation-specific multiplex ligation-dependent probe amplification and its impact on clinical findings in medulloblastoma. J Neurooncol 2013 doi:10.1007/s11060-11013-11286-11060.
Xie J, Murone M, Luoh S-M, Ryan A, Gu Q, Zhang C et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature 1998; 391: 90–92.
Mao J, Ligon KL, Rakhlin EY, Thayer SP, Bronson RT, Rowitch D et al. A novel somatic mouse model to survey tumorigenic potential applied to the hedgehog pathway. Cancer Res 2006; 66: 10171–10178.
Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP . Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev 2004; 18: 937–951.
Apte R, Dotan S, Elkabets M, White M, Reich E, Carmi Y et al. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metast Rev 2006; 25: 387–408.
Narayan S, Kolly L, So A, Busso N . Increased interleukin-10 production by ASC-deficient CD4+ T cells impairs bystander T-cell proliferation. Immunology 2011; 134: 33–40.
Ippagunta SK, Brand DD, Luo J, Boyd KL, Calabrese C, Stienstra R et al. Inflammasome-independent role of apoptosis-associated speck-like protein containing a CARD (ASC) in T cell priming is critical for collagen-induced arthritis. J Biol Chem 2010; 285: 12454–12462.
Kolly L, Karababa M, Joosten LA, Narayan S, Salvi R, Petrilli V et al. Inflammatory role of ASC in antigen-induced arthritis is independent of caspase-1, NALP-3, and IPAF. J Immunol 2009; 183: 4003–4012.
Shaw PJ, Lukens JR, Burns S, Chi H, McGargill MA, Kanneganti TD . Cutting edge: critical role for PYCARD/ASC in the development of experimental autoimmune encephalomyelitis. J Immunol 2010; 184: 4610–4614.
Drexler SK, Bonsignore L, Masin M, Tardivel A, Jackstadt R, Hermeking H et al. Tissue-specific opposing functions of the inflammasome adaptor ASC in the regulation of epithelial skin carcinogenesis. Proc Natl Acad Sci USA 2012; 109: 18384–18389.
Shibanuma M, Mashimo J, Mita A, Kuroki T, Nose K . Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-beta 1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur J Biochem 1993; 217: 13–19.
Shibanuma M, Nose K . Forced expression of hic-5, a senescence-related gene, potentiates a differentiation process of RCT-1 cells induced by retinoic acid. Int J Biochem Cell Biol 1998; 30: 39–45.
Dabiri G, Tumbarello DA, Turner CE, Van de Water L . TGF-beta1 slows the growth of pathogenic myofibroblasts through a mechanism requiring the focal adhesion protein, Hic-5. J Invest Dermatol 2008; 128: 280–291.
Northcott PA, Shih DJ, Peacock J, Garzia L, Morrissy AS, Zichner T et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature 2012; 488: 49–56.
Aref D, Moffatt CJ, Agnihotri S, Ramaswamy V, Dubuc AM, Northcott PA et al. Canonical TGF-beta pathway activity is a predictor of SHH-driven medulloblastoma survival and delineates putative precursors in cerebellar development. Brain Pathol 2012; 23: 178–191.
Cho YJ, Tsherniak A, Tamayo P, Santagata S, Ligon A, Greulich H et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol 2011; 29: 1424–1430.
Massague J . TGFbeta in Cancer. Cell 2008; 134: 215–230.
Rich JN . The role of transforming growth factor-beta in primary brain tumors. Front Biosci 2003; 8: e245–e260.
Rios I, Alvarez-Rodriguez R, Marti E, Pons S . Bmp2 antagonizes sonic hedgehog-mediated proliferation of cerebellar granule neurones through Smad5 signalling. Development 2004; 131: 3159–3168.
Zhao H, Ayrault O, Zindy F, Kim J-H, Roussel MF . Post-transcriptional down-regulation of Atoh1/Math1 by bone morphogenic proteins suppresses medulloblastoma development. Genes Dev 2008; 22: 722–727.
Liu W, Luo Y, Dunn JH, Norris DA, Dinarello CA, Fujita M . Dual role of apoptosis-associated speck-like protein containing a CARD (ASC) in tumorigenesis of human melanoma. J Invest Dermatol 2013; 133: 518–527.
Kole AJ, Swahari V, Hammond SM, Deshmukh M . miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes Dev 2011; 25: 125–130.
Kenney AM, Cole MD, Rowitch DH . Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development 2003; 130: 15–28.
Acknowledgements
We thank Drs Vishva Dixit (Genentech), James Olson (Fred Hutchinson Cancer Research Center), Eva Anton (UNC) for generously sharing ASC−/−, ND2:SmoA1 and hGFAP-cre mice, respectively. We appreciate the technical assistance provided by Vivian Xu, Michael Conlin and Meera Patel in the Deshmukh Lab; Janice Weaver, Lily Wai and Yongjuan Xia in the UNC Histopathology Core; Terese Camp and Ling Li in the UNC Genomics Core; Mark Vincent Olorvida, Stephanie Cohen and Bentley Midkiff in the UNC Translational Pathology Laboratory (TPL); and Joel Parker, George Wu, Chandri Yandava and Chris Fan at UNC for bioinformatics and biostatistics guidance. The UNC TPL is supported in part by grants from the National Cancer Institute (3P30CA016086) and the UNC University Cancer Research Fund. We would like to thank members of the Deshmukh Laboratory for critical review of this manuscript. TRG is supported by NIH Grant 1K08NS077978 and St Baldrick’s Foundation. This work was supported by Grants NS042197 and GM078366 to MD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Knight, E., Patel, E., Flowers, C. et al. ASC deficiency suppresses proliferation and prevents medulloblastoma incidence. Oncogene 34, 394–402 (2015). https://doi.org/10.1038/onc.2013.577
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.577
Keywords
This article is cited by
-
Comprehensive review of ASC structure and function in immune homeostasis and disease
Molecular Biology Reports (2020)
-
The IL-1/IL-1 receptor axis and tumor cell released inflammasome adaptor ASC are key regulators of TSLP secretion by cancer associated fibroblasts in pancreatic cancer
Journal for ImmunoTherapy of Cancer (2019)
-
NLR-regulated pathways in cancer: opportunities and obstacles for therapeutic interventions
Cellular and Molecular Life Sciences (2016)