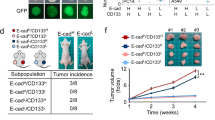
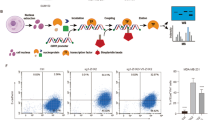
Abstract
Despite significant progress in the treatment of breast cancer, particularly through the use of targeted therapy, relapse and chemoresistance remain a major hindrance to the fight to minimize the burden of the disease. It is becoming increasingly clear that a rare subpopulation of cells known as cancer stem cells (CSC), able to be generated through epithelial-to-mesenchymal transition (EMT) and capable of tumor initiation and self-renewal, contributes to treatment resistance and metastases. This means that a more effective therapy should target both the chemoresistant CSCs and the proliferating epithelial cells that give rise to them to reverse EMT and to attenuate their conversion to CSCs. Here, we demonstrate a novel function of AXL in acting upstream to induce EMT in normal and immortalized human mammary epithelial cells in an apparent positive feedback loop mechanism and regulate breast CSC (BCSC) self-renewal and chemoresistance. Downregulation of AXL using MP470 (Amuvatinib) reversed EMT in mesenchymal normal human mammary epithelial cells and murine BCSCs attenuating self-renewal and restored chemosensitivity of the BCSCs. AXL expression was also found to be associated with the expression of stem cell genes, regulation of metastases genes, increased tumorigenicity and was important for BCSC invasion and migration. Inactivation of AXL also led to the downregulation of nuclear factor-κB pathway and reduced tumor formation in vivo. Taken together, our data suggest that targeted therapy against AXL, in combination with systemic therapies, has the potential to improve response to anticancer therapies and to reduce breast cancer recurrence and metastases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Han JS, Crowe DL . Tumor initiating cancer stem cells from human breast cancer cell lines. Int J Oncol 2009; 34: 1449–1453.
Vermeulen L, Sprick MR, Kemper K, Stassi G, Medema JP . Cancer stem cells—old concepts, new insights. Cell Death Differ 2008; 15: 947–958.
Vicente-Duenas C, Cobaleda C, Perez-Losada J, Sanchez-Garcia I . The evolution of cancer modeling: the shadow of stem cells. Dis Model Mech 2010; 3: 149–155.
Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, Kalli KR et al. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res 2009; 69: 2887–2895.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133: 704–715.
Radisky DC . Epithelial–mesenchymal transition. J Cell Sci 2005; 118: 4325–4326.
Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A . Generation of breast cancer stem cells through epithelial–mesenchymal transition. PLoS One 2008; 3: e2888.
Thiery JP, Acloque H, Huang RY, Nieto MA . Epithelial–mesenchymal transitions in development and disease. Cell 2009; 139: 871–890.
Thiery JP . Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2: 442–454.
Cowin P, Welch DR . Breast cancer progression: controversies and consensus in the molecular mechanisms of metastasis and EMT. J Mammary Gland Biol Neoplasia 2007; 12: 99–102.
Lee JM, Dedhar S, Kalluri R, Thompson EW . The epithelial–mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 2006; 172: 973–981.
O’Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C et al. Axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol 1991; 11: 5016–5031.
Bose R, Molina H, Patterson AS, Bitok JK, Periaswamy B, Bader JS et al. Phosphoproteomic analysis of Her2/neu signaling and inhibition. Proc Natl Acad Sci USA 2006; 103: 9773–9778.
Hafizi S, Dahlback B . Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J 2006; 273: 5231–5244.
Hutterer M, Knyazev P, Abate A, Reschke M, Maier H, Stefanova N et al. Axl and growth arrest-specific gene 6 are frequently overexpressed in human gliomas and predict poor prognosis in patients with glioblastoma multiforme. Clin Cancer Res 2008; 14: 130–138.
Li Y, Ye X, Tan C, Hongo JA, Zha J, Liu J et al. Axl as a potential therapeutic target in cancer: role of Axl in tumor growth, metastasis and angiogenesis. Oncogene 2009; 28: 3442–3455.
Zhang YX, Knyazev PG, Cheburkin YV, Sharma K, Knyazev YP, Orfi L et al. AXL is a potential target for therapeutic intervention in breast cancer progression. Cancer Res 2008; 68: 1905–1915.
Gjerdrum C, Tiron C, Høiby T, Stefansson I, Haugen H, Sandal T et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci USA 2010; 107: 1124–1129.
Liu L, Greger J, Shi H, Liu Y, Greshock J, Annan R et al. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res 2009; 69: 6871–6878.
Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009; 138: 645–659.
Asiedu MK, Ingle JN, Behrens MD, Radisky DC, Knutson KL . TGF{beta}/TNF{alpha}-mediated epithelial–mesenchymal transition generates breast cancer stem cells with a claudin-low phenotype. Cancer Res 2011; 71: 4707–4719.
Qi W, Cooke LS, Stejskal A, Riley C, Croce KD, Saldanha JW et al. MP470, a novel receptor tyrosine kinase inhibitor, in combination with Erlotinib inhibits the HER family/PI3K/Akt pathway and tumor growth in prostate cancer. BMC Cancer 2009; 9: 142.
Welsh JW, Mahadevan D, Ellsworth R, Cooke L, Bearss D, Stea B . The c-Met receptor tyrosine kinase inhibitor MP470 radiosensitizes glioblastoma cells. Radiat Oncol 2009; 4: 69.
Meng F, Liu L, Chin PC, D’Mello SR . Akt is a downstream target of NF-kappa B. J Biol Chem 2002; 277: 29674–29680.
Tai KY, Shieh YS, Lee CS, Shiah SG, Wu CW . Axl promotes cell invasion by inducing MMP-9 activity through activation of NF-kappaB and Brg-1. Oncogene 2008; 27: 4044–4055.
Chen W, Li Z, Bai L, Lin Y . NF-kappaB in lung cancer, a carcinogenesis mediator and a prevention and therapy target. Front Biosci 2011; 16: 1172–1185.
Shipitsin M, Polyak K . The cancer stem cell hypothesis: in search of definitions, markers, and relevance. Lab Invest 2008; 88: 459–463.
Wicha MS . Cancer stem cells and metastasis: lethal seeds. Clin Cancer Res 2006; 12: 5606–5607.
Gangemi R, Paleari L, Orengo AM, Cesario A, Chessa L, Ferrini S et al. Cancer stem cells: a new paradigm for understanding tumor growth and progression and drug resistance. Curr Med Chem 2009; 16: 1688–1703.
Gupta PB, Chaffer CL, Weinberg RA . Cancer stem cells: mirage or reality? Nat Med 2009; 15: 1010–1012.
Blick T, Hugo H, Widodo E, Waltham M, Pinto C, Mani SA et al. Epithelial–mesenchymal transition traits in human breast cancer cell lines parallel the CD44(hi/)CD24 (lo/−) stem cell phenotype in human breast cancer. J Mammary Gland Biol Neoplasia 2010; 15: 235–252.
Rankin EB, Fuh KC, Taylor TE, Krieg AJ, Musser M, Yuan J et al. AXL is an essential factor and therapeutic target for metastatic ovarian cancer. Cancer Res 2010; 70: 7570–7579.
Nakano T, Tani M, Ishibashi Y, Kimura K, Park YB, Imaizumi N et al. Biological properties and gene expression associated with metastatic potential of human osteosarcoma. Clin Exp Metast 2003; 20: 665–674.
Vajkoczy P, Knyazev P, Kunkel A, Capelle HH, Behrndt S, von Tengg-Kobligk H et al. Dominant-negative inhibition of the Axl receptor tyrosine kinase suppresses brain tumor cell growth and invasion and prolongs survival. Proc Natl Acad Sci USA 2006; 103: 5799–5804.
Shieh YS, Lai CY, Kao YR, Shiah SG, Chu YW, Lee HS et al. Expression of axl in lung adenocarcinoma and correlation with tumor progression. Neoplasia 2005; 7: 1058–1064.
Chung BI, Malkowicz SB, Nguyen TB, Libertino JA, McGarvey TW . Expression of the proto-oncogene Axl in renal cell carcinoma. DNA Cell Biol 2003; 22: 533–540.
van Ginkel PR, Gee RL, Shearer RL, Subramanian L, Walker TM, Albert DM et al. Expression of the receptor tyrosine kinase Axl promotes ocular melanoma cell survival. Cancer Res 2004; 64: 128–134.
Craven RJ, Xu LH, Weiner TM, Fridell YW, Dent GA, Srivastava S et al. Receptor tyrosine kinases expressed in metastatic colon cancer. Int J Cancer 1995; 60: 791–797.
Song X, Wang H, Logsdon CD, Rashid A, Fleming JB, Abbruzzese JL et al. Overexpression of receptor tyrosine kinase Axl promotes tumor cell invasion and survival in pancreatic ductal adenocarcinoma. Cancer 117: 734–743.
Vuoriluoto K, Haugen H, Kiviluoto S, Mpindi JP, Nevo J, Gjerdrum C et al. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene 2011; 30: 1436–1448.
Thiery JP, Sleeman JP . Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol 2006; 7: 131–142.
Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004; 117: 927–939.
Koorstra JB, Karikari CA, Feldmann G, Bisht S, Rojas PL, Offerhaus GJ et al. The Axl receptor tyrosine kinase confers an adverse prognostic influence in pancreatic cancer and represents a new therapeutic target. Cancer Biol Ther 2009; 8: 618–626.
Lee WP, Liao Y, Robinson D, Kung HJ, Liu ET, Hung MC . Axl–gas6 interaction counteracts E1A-mediated cell growth suppression and proapoptotic activity. Mol Cell Biol 1999; 19: 8075–8082.
Holland SJ, Pan A, Franci C, Hu Y, Chang B, Li W et al. R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res 2010; 70: 1544–1554.
Keating AK, Kim GK, Jones AE, Donson AM, Ware K, Mulcahy JM et al. Inhibition of Mer and Axl receptor tyrosine kinases in astrocytoma cells leads to increased apoptosis and improved chemosensitivity. Mol Cancer Ther 2010; 9: 1298–1307.
Grimshaw MJ, Cooper L, Papazisis K, Coleman JA, Bohnenkamp HR, Chiapero-Stanke L et al. Mammosphere culture of metastatic breast cancer cells enriches for tumorigenic breast cancer cells. Breast Cancer Res 2008; 10: R52.
Acknowledgements
This work was supported by a generous gift from Martha and Bruce Atwater (KLK); Howard Temin Award, K01-CA100764 (KLK); R01-CA122086 (DCR); and the Mayo Clinic Breast Cancer Specialized Program of Research Excellence Award, P50-CA116201 (JNI). This publication was also supported by NIH/NCRR CTSA Grant Number UL1 RR024150. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. We acknowledge the strong support of the Mayo Clinic Comprehensive Cancer Center for providing access to core facilities.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Asiedu, M., Beauchamp-Perez, F., Ingle, J. et al. AXL induces epithelial-to-mesenchymal transition and regulates the function of breast cancer stem cells. Oncogene 33, 1316–1324 (2014). https://doi.org/10.1038/onc.2013.57
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.57
Keywords
This article is cited by
-
Mesenchymal–epithelial transition and AXL inhibitor TP-0903 sensitise triple-negative breast cancer cells to the antimalarial compound, artesunate
Scientific Reports (2024)
-
NF-κB signaling in neoplastic transition from epithelial to mesenchymal phenotype
Cell Communication and Signaling (2023)
-
Therapeutic targeting of the functionally elusive TAM receptor family
Nature Reviews Drug Discovery (2023)
-
The effect of inhibition of receptor tyrosine kinase AXL on DNA damage response in ovarian cancer
Communications Biology (2023)
-
NRG1 regulates Fra-1 transcription and metastasis of triple-negative breast cancer cells via the c-Myc ubiquitination as manipulated by ERK1/2-mediated Fbxw7 phosphorylation
Oncogene (2022)