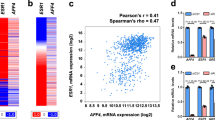
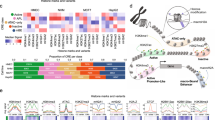
Abstract
The estrogen receptor alpha (ERα) is the central transcriptional regulator of ductal mammary epithelial lineage specification and is an important prognostic marker in human breast cancer. Although antiestrogen therapies are initially highly effective at treating ERα-positive tumors, a large number of tumors progress to a refractory, more poorly differentiated phenotype accompanied by reduced survival. A better understanding of the molecular mechanisms involved in the progression from estrogen-dependent to hormone-resistant breast cancer may uncover new targets for treatment and the discovery of new predictive markers. Recent studies have uncovered an important role for transcriptional elongation and chromatin modifications in controlling ERα activity and estrogen responsiveness. The human Suppressor of Ty Homologue-6 (SUPT6H) is a histone chaperone that links transcriptional elongation to changes in chromatin structure. We show that SUPT6H is required for estrogen-regulated transcription and the maintenance of chromatin structure in breast cancer cells, possibly in part through interaction with RNF40 and regulation of histone H2B monoubiquitination (H2Bub1). Moreover, we demonstrate that SUPT6H protein levels decrease with malignancy in breast cancer. Consistently, SUPT6H, similar to H2Bub1, is required for cellular differentiation and suppression of the repressive histone mark H3K27me3 on lineage-specific genes. Together, these data identify SUPT6H as a new epigenetic regulator of ERα activity and cellular differentiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Deroo BJ, Korach KS . Estrogen receptors and human disease. J Clin Invest 2006; 116: 561–570.
Ali S, Coombes RC . Estrogen receptor alpha in human breast cancer: occurrence and significance. J Mammary Gland Biol Neoplasia 2000; 5: 271–281.
Khan SA, Rogers MAM, Khurana KK, Meguid MM, Numann PJ . Estrogen receptor expression in benign breast epithelium and breast cancer risk. JNCI J Natl Cancer Inst 1998; 90: 37–42.
Theodorou V, Stark R, Menon S, Carroll JS . GATA3 acts upstream of FOXA1 in mediating ESR1 binding by shaping enhancer accessibility. Genome Res 2013; 23: 12–22.
Kininis M, Isaacs GD, Core LJ, Hah N, Kraus WL . Postrecruitment regulation of RNA polymerase II directs rapid signaling responses at the promoters of estrogen target genes. Mol Cell Biol 2009; 29: 1123–1133.
Wittmann BM, Fujinaga K, Deng H, Ogba N, Montano MM . The breast cell growth inhibitor, estrogen down regulated gene 1, modulates a novel functional interaction between estrogen receptor alpha and transcriptional elongation factor cyclin T1. Oncogene 2005; 24: 5576–5588.
Egloff S, Murphy S . Cracking the RNA polymerase II CTD code. Trends Genet 2008; 24: 280–288.
Ogba N, Chaplin LJ, Doughman YQ, Fujinaga K, Montano MM . HEXIM1 regulates 17β-estradiol/estrogen receptor-α–mediated expression of cyclin d1 in mammary cells via modulation of P-TEFb. Cancer Res 2008; 68: 7015–7024.
Ketchart W, Ogba N, Kresak A, Albert JM, Pink JJ, Montano MM . HEXIM1 is a critical determinant of the response to tamoxifen. Oncogene 2011; 30: 3563–3569.
Kouzarides T . Chromatin modifications and their function. Cell 2007; 128: 693–705.
Li B, Carey M, Workman JL . The role of chromatin during transcription. Cell 2007; 128: 707–719.
Fullwood MJ, Liu MH, Pan YF, Liu J, Xu H, Mohamed YB et al. An oestrogen-receptor-alpha-bound human chromatin interactome. Nature 2009; 462: 58–64.
Métivier R, Reid G, Gannon F . Transcription in four dimensions: nuclear receptor-directed initiation of gene expression. EMBO Rep 2006; 7: 161–167.
Johnsen SA, Kangaspeska S, Reid G, Gannon F . Interfering with the dynamics of estrogen receptor-regulated transcription. Ernst Schering Found Symp Proc 2006; 1: 1–12.
Prenzel T, Begus-Nahrmann Y, Kramer F, Hennion M, Hsu C, Gorsler T et al. Estrogen-dependent gene transcription in human breast cancer cells relies upon proteasome-dependent monoubiquitination of histone H2B. Cancer Res 2011; 71: 5739–5753.
Shema E, Tirosh I, Aylon Y, Huang J, Ye C, Moskovits N et al. The histone H2B-specific ubiquitin ligase RNF20/hBRE1 acts as a putative tumor suppressor through selective regulation of gene expression. Genes Dev 2008; 22: 2664–2676.
Johnsen SA . The enigmatic role of H2Bub1 in cancer. FEBS Lett 2012; 586: 1592–1601.
Karpiuk O, Najafova Z, Kramer F, Hennion M, Galonska C, König A et al. The histone H2B monoubiquitination regulatory pathway is required for differentiation of multipotent stem cells. Mol Cell 2012; 46: 705–713.
Fuchs G, Shema E, Vesterman R, Kotler E, Wolchinsky Z, Wilder S et al. RNF20 and USP44 regulate stem cell differentiation by modulating H2B monoubiquitylation. Mol Cell 2012; 46: 662–673.
Chen S, Li J, Wang D-L, Sun F-L . Histone H2B lysine 120 monoubiquitination is required for embryonic stem cell differentiation. Cell Res 2012; 22: 1402–1405.
Minsky N, Shema E, Field Y, Schuster M, Segal E, Oren M . Monoubiquitinated H2B is associated with the transcribed region of highly expressed genes in human cells. Nature Cell Biol 2008; 10: 483–488.
Pirngruber J, Shchebet A, Schreiber L, Shema E, Minsky N, Chapman RD et al. CDK9 directs H2B monoubiquitination and controls replication-dependent histone mRNA 3′-end processing. EMBO Rep 2009; 10: 894–900.
Johnsen SA . CDK9 and H2B monoubiquitination: a well-choreographed dance. PLoS Genet 2012; 8: e1002860.
Zhang F, Yu X . WAC, a functional partner of RNF20/40, regulates histone H2B ubiquitination and gene transcription. Mol Cell 2011; 41: 384–397.
Yoh SM, Cho H, Pickle L, Evans RM, Jones KA . The Spt6 SH2 domain binds Ser2-P RNAPII to direct Iws1-dependent mRNA splicing and export. Genes Dev 2007; 21: 160–174.
Diebold M-L, Loeliger E, Koch M, Winston F, Cavarelli J, Romier C . Noncanonical tandem SH2 enables interaction of elongation factor Spt6 with RNA polymerase II. J Biol Chem 2010; 285: 38389–38398.
Baniahmad C, Nawaz Z, Baniahmad A, Gleeson MA, Tsai MJ, O’Malley BW . Enhancement of human estrogen receptor activity by SPT6: a potential coactivator. Mol Endocrinol 1995; 9: 34–43.
Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet 2006; 38: 1289–1297.
Bortvin A, Winston F . Evidence that Spt6p controls chromatin structure by a direct interaction with histones. Science 1996; 272: 1473–1476.
Kim J, Guermah M, McGinty RK, Lee J-S, Tang Z, Milne TA et al. RAD6-Mediated transcription-coupled H2B ubiquitylation directly stimulates H3K4 methylation in human cells. Cell 2009; 137: 459–471.
Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med 2008; 14: 518–527.
Richardson AL, Wang ZC, De Nicolo A, Lu X, Brown M, Miron A et al. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell 2006; 9: 121–132.
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001; 98: 10869–10874.
Abd El-Rehim DM, Pinder SE, Paish CE, Bell J, Blamey R, Robertson JF et al. Expression of luminal and basal cytokeratins in human breast carcinoma. J Pathol 2004; 203: 661–671.
Kilpinen S, Autio R, Ojala K, Iljin K, Bucher E, Sara H et al. Systematic bioinformatic analysis of expression levels of 17 330 human genes across 9783 samples from 175 types of healthy and pathological tissues. Genome Biol 2008; 9: R139.
Simonsen JL, Rosada C, Serakinci N, Justesen J, Stenderup K, Rattan SIS et al. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotech 2002; 20: 592–596.
Collett K, Eide GE, Arnes J, Stefansson IM, Eide J, Braaten A et al. Expression of enhancer of zeste homologue 2 is significantly associated with increased tumor cell proliferation and is a marker of aggressive breast cancer. Clin Cancer Res 2006; 12: 1168–1174.
Raaphorst FM, CJLM Meijer, Fieret E, Blokzijl T, Mommers E, Buerger H et al. Poorly differentiated breast carcinoma is associated with increased expression of the human polycomb group EZH2 gene. Neoplasia 2003; 5: 481–488.
Reijm EA, Jansen MPHM, Ruigrok-Ritstier K, van Staveren IL, Look MP, van Gelder MEM et al. Decreased expression of EZH2 is associated with upregulation of ER and favorable outcome to tamoxifen in advanced breast cancer. Breast Cancer Res Treat 2011; 125: 387–394.
Chen S, Ma J, Wu F, Xiong L-J, Ma H, Xu W et al. The histone H3 Lys 27 demethylase JMJD3 regulates gene expression by impacting transcriptional elongation. Genes Dev 2012; 26: 1364–1375.
Wang AH, Zare H, Mousavi K, Wang C, Moravec CE, Sirotkin HI et al. The histone chaperone Spt6 coordinates histone H3K27 demethylation and myogenesis. EMBO J 2013; 32: 1075–1086.
Pirngruber J, Shchebet A, Johnsen SA . Insights into the function of the human P-TEFb component CDK9 in the regulation of chromatin modifications and co-transcriptional mRNA processing. Cell Cycle 2009; 8: 3636–3642.
Pirngruber J, Johnsen SA . Induced G1 cell-cycle arrest controls replication-dependent histone mRNA 3′ end processing through p21, NPAT and CDK9. Oncogene 2010; 29: 2853–2863.
Kari V, Shchebet A, Neumann H, Johnsen SA . The H2B ubiquitin ligase RNF40 cooperates with SUPT16H to induce dynamic changes in chromatin structure during DNA double-strand break repair. Cell Cycle 2011; 10: 3495–3504.
Schneider CA, Rasband WS, Eliceiri KW . NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9: 671–675.
Ruifrok AC, Johnston DA . Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol 2001; 23: 291–299.
Acknowledgements
We thank L Schreiber, J Schiller and CR Schlegel for initial efforts in establishing SUPT6H knockdown. UB was supported by doctoral fellowships from the Erasmus Mundus External Cooperation Window EURINDIA and the Göttingen Graduate School for Neurosciences, Biophysics and Molecular Biosciences. This work was supported by grants from the Deutsche Krebshilfe (109088) and the Deutsche Forschungsgemeinschaft (JO 815/3) to SAJ.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Bedi, U., Scheel, A., Hennion, M. et al. SUPT6H controls estrogen receptor activity and cellular differentiation by multiple epigenomic mechanisms. Oncogene 34, 465–473 (2015). https://doi.org/10.1038/onc.2013.558
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.558
Keywords
This article is cited by
-
Survival is associated with repressive histone trimethylation markers in both HR-positive HER2-negative and triple-negative breast cancer patients
Virchows Archiv (2023)
-
Estrogen receptor beta repurposes EZH2 to suppress oncogenic NFκB/p65 signaling in triple negative breast cancer
npj Breast Cancer (2022)
-
RNF40 exerts stage-dependent functions in differentiating osteoblasts and is essential for bone cell crosstalk
Cell Death & Differentiation (2021)
-
Epigenetic modification and a role for the E3 ligase RNF40 in cancer development and metastasis
Oncogene (2021)
-
The histone H2B ubiquitin ligase RNF40 is required for HER2-driven mammary tumorigenesis
Cell Death & Disease (2020)