Abstract
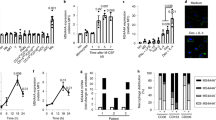
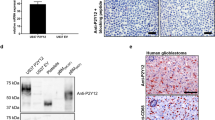
Macrophage polarization contributes to distinct human pathologies. In tumors, a polarized M2 phenotype called tumor-associated macrophages (TAMs) are associated with promotion of invasion and angiogenesis. In cancer cells, vacuolar ATPase (V-ATPase), a multi-subunit enzyme, is expressed on the plasma/vesicular membranes and critically influences the metastatic behavior. In addition, the soluble, cleaved N-terminal domain of a2 isoform of V-ATPase (a2NTD) is associated with in vitro induction of pro-tumorigenic properties in monocytes. This activity of a2 isoform of V-ATPase (a2V) caused us to investigate its role in cancer progression through the evaluation of the immunomodulatory properties of a2NTD. Here, we present direct evidence that surface expression of V-ATPase is associated with macrophage polarization in tumor tissue. Macrophages from BALB/c mice (peritoneal/bone marrow derived) were stimulated with recombinant a2NTD in both ex vivo and in vivo systems and evaluated for TAM characteristics. a2V was highly expressed in tumor tissues (breast and skin) as well as on the surface of tumor cell lines. The a2NTD-stimulated macrophages (a2MΦ) acquired TAM phenotype, which was characterized by elevated expression of mannose receptor-1, Arginase-1, interleukin-10 and transforming growth factor-β. a2MΦ also exhibited increased production of other tumorigenic factors including matrix metalloproteinase-9 and vascular endothelial growth factor. Further, a2MΦ were cocultured with mouse B-16F0 melanoma cells for their functional characterization. The coculture of these a2MΦ subsequently increased the invasion and angiogenesis of less invasive B-16F0 cells. When cocultured with naive T cells, a2MΦ significantly inhibited T-cell activation. The present data establish the role of V-ATPase in modulating a macrophage phenotype towards TAMs through the action of a2NTD, suggesting it to be a potential therapeutic target in cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sennoune SR, Luo D, Martínez-Zaguilán R . Plasmalemmal vacuolar-type H+-ATPase in cancer biology. Cell Biochem Biophys 2004; 40: 185–206.
Torigoe T, Izumi H, Ishiguchi H, Uramoto H, Murakami T, Ise T et al. Enhanced expression of the human vacuolar Hþ-ATPase c subunit gene (ATP6L) in response to anticancer agents. J Biol Chem 2002; 277: 36534–36543.
Sennoune SR, Bakunts K, Martínez GM, Chua-Tuan JL, Kebir Y, Attaya MN et al. Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am J Physiol Cell Physiol 2004; 286: C1443–C1452.
Ntrivalas E, Gilman-Sachs A, Kwak-Kim J, Beaman K . The N-terminus domain of the a2 isoform of vacuolar ATPase can regulate interleukin- 1b production from mononuclear cells in coculture with JEG-3 choriocarcinoma cells. Am J Reprod Immunol 2007; 57: 201–209.
Ntrivalas E, Derks R, Gilman-Sachs A, Kwak-Kim J, Levine R, Beaman K . Novel role for the N-terminus domain of the a2 isoform of vacuolar ATPase in interleukin-1b production. Hum Immunol 2007; 68: 469–477.
Kwong C, Gilman-Sachs A, Beaman K . Tumor-associated a2 vacuolar ATPase acts as a key mediator of cancer-related inflammation by inducing pro-tumorigenic properties in monocytes. J Immunol 2011; 186: 1781–1789.
Jaiswal MK, Mallers TM, Larsen B, Kwak-Kim J, Chaouat G, Gilman-Sachs A et al. V-ATPase upregulation during early pregnancy: a possible link to establishment of an inflammatory response during preimplantation period of pregnancy. Reproduction 2012; 143: 713–725.
Kwong C, Gilman-Sachs A, Beaman K . An independent endocytic pathway stimulates different monocyte subsets by the a2 N-terminus domain of vacuolar-ATPase. Oncoimmunology 2013; 2: e22978.
Watkins SK, Egilmez NK, Suttles J, Stout RD . IL-12 rapidly alter the functional profiles of tumor-associated and tumor-infiltrating macrophages in vitro and in vivo. J Immunol 2007; 178: 1357–1362.
Martinez FO, Gordon S, Locati M, Manotvani A . Transcriptional profiling of the human monocyte to macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 2006; 199: 7303–7311.
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M . The chemokine systems in diverse forms of macrophage activation and polarization. Trends Immunol 2004; 25: 677–686.
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A . Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002; 23: 549–555.
Sica A, Schioppa T, Mantovani A, Allavena P . Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer 2006; 42: 717–727.
Hao NB, Lü MH, Fan YH, Cao YL, Zhang ZR, Yang SM . Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol 2012; 2012: 948098.
Farber MJ, Rizaldy R, Hildebrand JD . Shroom2 regulates contractility to control endothelial morphogenesis. Mol Biol Cell 2011; 22: 795–805.
Martínez-Zaguilán R, Lynch RM, Martinez GM, Gillies RJ . Vacuolar-type H_-ATPases are functionally expressed in plasma membranes of human tumor cells. Am J Physiol Cell Physiol 1993; 265: C1015–C1029.
Forgac M . Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 2007; 8: 917–929.
Hinton A, Sennoune SR, Bond S, Fang M, Reuveni M, Sahagian GG et al. Function of a subunit isoforms of the V-ATPase in pH homeostasis and in vitro invasion of MDA-MB231 human breast cancer cells. J Biol Chem 2009; 284: 16400–16408.
Nishisho T, Hata K, Nakanishi M, Morita Y, Sun-Wada GH, Wada Y et al. The a3 isoform vacuolar type H^-ATPase promotes distant metastasis in the mouse B16 melanoma cells. Mol Cancer Res 2011; 9: 845–855.
De Milito A, Marino ML, Fais S . A rationale for the use of proton pump inhibitors as antineoplastic agents. Curr Pharm Des 2012; 18: 1395–1406.
Gottesman MM, Fojo T, Bates SE . Multidrug resistance in cancer: role of ATP dependent transporters. Nat Rev Cancer 2002; 2: 48–58.
Lu X, Qin W, Li J, Tan N, Pan D, Zhang H et al. The growth and metastasis of human hepatocellular carcinoma xenografts are inhibited by small interfering RNA targeting to the subunit ATP6L of proton pump. Cancer Res 2005; 65: 6843–6849.
You H, Jin J, Shu H, Yu B, De Milito A, Lozupone F et al. Small interfering RNA targeting the subunit ATP6L of proton pump V-ATPase overcomes chemoresistance of breast cancer cells. Cancer Lett 2009; 280: 110–119.
Matlack R, Yeh K, Rosini L, Gonzalez D, Taylor J, Silberman D et al. Peritoneal macrophages suppress T-cell activation by amino acid catabolism. Immunology 2006; 117: 386–395.
Derks R, Beaman K . Regeneration and tolerance factor modulates the effect of adenosine triphosphate-induced interleukin 1b secretion in human macrophages. Hum Immunol 2004; 65: 676–682.
Acknowledgements
We thank Robert Dickinson for technical assistance and the Rosalind Franklin University of Medicine and Science Flow Cytometry Core Facility. This work was supported by grants from the Clinical Immunology Laboratory, Rosalind Franklin University of Medicine and Science, North Chicago, IL, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Katara, G., Jaiswal, M., Kulshrestha, A. et al. Tumor-associated vacuolar ATPase subunit promotes tumorigenic characteristics in macrophages. Oncogene 33, 5649–5654 (2014). https://doi.org/10.1038/onc.2013.532
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.532
Keywords
This article is cited by
-
SEVs-mediated miR-6750 transfer inhibits pre-metastatic niche formation in nasopharyngeal carcinoma by targeting M6PR
Cell Death Discovery (2023)
-
Cancer derived peptide of vacuolar ATPase ‘a2’ isoform promotes neutrophil migration by autocrine secretion of IL-8
Scientific Reports (2016)
-
Inhibition of vacuolar ATPase subunit in tumor cells delays tumor growth by decreasing the essential macrophage population in the tumor microenvironment
Oncogene (2016)
-
Future directions of clinical laboratory evaluation of pregnancy
Cellular & Molecular Immunology (2014)
-
Microenvironmental acidosis in carcinogenesis and metastases: new strategies in prevention and therapy
Cancer and Metastasis Reviews (2014)