Abstract
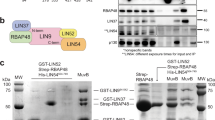
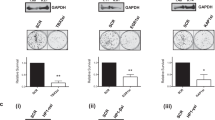
The Wilms’ tumor-1 protein (WT1) is a transcriptional regulator that can either activate or repress genes controlling cell growth, apoptosis and differentiation. The transcriptional corepressor BASP1 interacts with WT1 and mediates WT1’s transcriptional repression activity. BASP1 is contained within large complexes, suggesting that it works in concert with other factors. Here we report that the transcriptional repressor prohibitin is part of the WT1–BASP1 transcriptional repression complex. Prohibitin interacts with BASP1, colocalizes with BASP1 in the nucleus, and is recruited to the promoter region of WT1 target genes to elicit BASP1-dependent transcriptional repression. We demonstrate that prohibitin and BASP1 cooperate to recruit the chromatin remodeling factor BRG1 to WT1-responsive promoters and that this results in the dissociation of CBP from the promoter region of WT1 target genes. As seen with BASP1, prohibitin can associate with phospholipids. We demonstrate that the recruitment of PIP2 and HDAC1 to WT1 target genes is also dependent on the concerted activity of BASP1 and prohibitin. Our findings provide new insights into the function of prohibitin in transcriptional regulation and uncover a BASP1–prohibitin complex that plays an essential role in the PIP2-dependent recruitment of chromatin remodeling activities to the promoter.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rivera MN, Haber DA . Wilms’ tumour: connecting tumorigenesis and organ development in the kidney. Nat Rev Cancer 2005; 5: 699–712.
Hohenstein P, Hastie ND . The many facets of the Wilms’ tumour gene, WT1. Hum Mol Genet 2006; 15 (Spec No 2): R196–R201.
Huff V . Wilms’ tumours: about tumour suppressor genes, an oncogene and a chameleon gene. Nat Rev Cancer 2011; 11: 111–121.
Roberts SGE . Transcriptional regulation by WT1 in development. Curr Opin Genet Dev 2005; 15: 542–547.
McKay LM, Carpenter B . Roberts SGE. Regulation of the Wilms’ tumour suppressor protein transcriptional activator domain. Oncogene 1999; 18: 6546–6554.
Carpenter B, Hill KJ, Charalambous M, Wagner KJ, Lahiri D, James DI et al. BASP1 is a transcriptional cosuppressor for the Wilms’ tumor suppressor protein WT1. Mol Cell Biol 2004; 24: 537–549.
Mosevitsky MI . Nerve ending ‘signal’ proteins GAP-43, MARCKS and BASP1. Int Rev Cytol 2005; 245: 245–325.
Green LM, Wagner KJ, Campbell HA, Addison K . Roberts SGE. Dynamic interaction between WT1 and BASP1 in transcriptional regulation during differentiation. Nucleic Acids Res 2009; 37: 431–440.
Essafi A, Webb A, Berry RL, Slight J, Burn SF, Spraggon L et al. A Wt1-controlled chromatin switching mechanism underpins tissue-specific wnt4 activation and repression. Dev Cell 2011; 21: 559–574.
Goodfellow SJ, Rebello MR, Toska E, Zeef LA, Rudd SG, Medler KF et al. WT1 and its transcriptional cofactor BASP1 redirect the differentiation pathway of an established blood cell line. Biochem J 2011; 435: 113–125.
Toska E, Campbell HA, Shandilya J, Goodfellow SJ, Shore P, Medler KF et al. Repression of transcription by WT1-BASP1 requires the myristoylation of BASP1 and the PIP2-dependent recruitment of histone deacetylase. Cell Reports 2012; 2: 462–469.
Hartl M, Nist A, Khan MI, Valovka T, Bister K . inhibition of Myc-induced cell transformation by brain acid-soluble protein 1 (BASP1). Proc Natl Acad Sci USA 2009; 106: 5604–5609.
Yeoh EJ, Ross ME, Shurtleff SA, Williams WK, Patel D, Mahfouz R et al. Classification, subtype discovery and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expressing profiling. Cancer Cell 2002; 1: 133–143.
Moribe T, Lizuka N, Miura T, Stark M, Tamatsukuri S, Ishitsuka H et al. Identification of novel aberrant methylation of BASP1 and SRD5A2 for early diagnosis of hepatocellular carcinoma by genome-wide search. Int J Oncol 2008; 33: 949–958.
Wang S, Nath N, Adlam M, Chellappan S . Prohibitin, a potential tumor suppressor, interacts with RB and regulates E2F function. Oncogene 1999; 18: 3501–3510.
Wang S, Nath N, Fusaro G, Chellappan S . Rb and prohibitin target distinct regions of E2F1 for repression and respond to different upstream signals. Mol Cell Biol 1999; 19: 7447–7460.
Wang S, Zhang B, Faller DV . Prohibitin requires Brg-1 and Brm for the repression of E2F and cell growth. EMBO J 2002; 21: 3019–3028.
Joshi B, Ko D, Ordonez-Ercan D, Chellappan SP . A putative coiled-coil domain of prohibitin is sufficient to repress E2F1-mediated transcriptional and induce apoptosis. Biochem Biophys Res Commun 2003; 312: 459–466.
Choi D, Lee SJ, Hong S, Kim IH, Kang S . Prohibitin interacts with RNF2 and regulates E2F1 function via dual pathways. Oncogene 2008; 27: 1716–1725.
Schneider M, Schambony A, Wedlich D . Prohibitin1 acts as a neural crest specifier in Xenopus development by repressing the transcription factor E2F1. Development 2010; 137: 4073–4081.
Wang S, Fusaro G, Padmanabhan J, Chellappan SP . Prohibitin co-localizes with Rb in the nucleus and recruits N-CoR and HDAC1 for transcriptional repression. Oncogene 2002; 21: 8388–8396.
Montano MM, Ekena K, Delage-Mourroux R, Chang W, Martini P, Katzenellenbogen BS . An estrogen receptor-selective coregulator that potentiates the effectiveness of aniestrogenes and represses the activity of estrogens. Proc Natl Acad Sci USA 1999; 96: 6947–6952.
Delage-Mourroux R, Martini PG, Choi I, Kraichely DM, Hoeksema J, Katzenellenbogen BS . Analysis of estrogen receptor interaction with a repressor of estrogen receptor activity (REA) and the regulation of estrogen receptor transcriptional activity by REA. J Biol Chem 2000; 275: 35848–35856.
He B, Feng Q, Mukherjee A, Lonard DM, DeMayo FJ, Katzenellenbogen BS et al. A repressive role for prohibitin in estrogen signaling. Mol Endocrinol 2008; 22: 344–360.
Gamble SC, Chotai D, Odontiadis M, Dart DA, Brooke GN, Powell SM et al. Prohibitin, a protein downregulated by androgens, represses androgen receptor activity. Oncogene 2007; 26: 1757–1768.
Dai Y, Ngo D, Jacob J, Forman LW, Faller DV . Prohibitin and the SW1/SNF ATPase subunit BRG1 are required for effective androgen antagonist-mediated transcriptional repression of androgen receptor regulated genes. Carcinogenesis 2008; 29: 1725–1733.
Mishra S, Murphy LC, Murphy LJ . The prohibitins: emerging roles in diverse functions. J Cell Mol Med 2006; 10: 353–363.
Kim HS, Kim MS, Hancock AL, Harper JCP, Park JY, Poy G et al. Identification of novel Wilms’ tumor suppressor gene target genes implicated in kidney development. J Biol Chem 2007; 282: 16278–16287.
Hartkamp J, Carpenter B, Roberts SGE . The Wilms’ tumor suppressor protein WT1 is processed by the serine protease HtrA2/Omi. Mol Cell 2010; 37: 159–171.
Wang S, Zhang B, Faller DV . BRG1/BRM and prohibitin are required for growth suppression by estrogen antagonists. EMBO J 2004; 23: 2293–2303.
Zhang B, Chambers Kj, Faller DV, Wang S . Reprogramming of the SWI/SNF complex for coactivation or co-repression in prohibitin-mediated estrogen receptor regulation. Oncogene 2007; 26: 7153–7157.
Havas K, Flaus A, Phelan M, Kingston R, Wade PA, Lilley DM et al. Generation of superhelical torsion by ATP-dependent chromatin remodeling activities. Cell 2000; 103: 1133–1142.
Kadam S, Emerson BM . Transcriptional specificity of human SWI/SNF BRG1 and BRM chromatin remodeling complexes. Mol Cell 2003; 11: 377–389.
Sif S, Saurin AJ, Imbalzano AN, Kingston RE . Purification and characterization of mSin3A-containing Brg1 and hBrm chromatin remodeling complexes. Genes Dev 2001; 15: 603–618.
Trotter KW, Archer TK . The BRG1 transcriptional coregulator. Nucl Receptor Signaling 2008; 6: e004.
Ande SR, Mishra S . Prohibitin interacts with phosphatidylinositol 3,4,5-triphosphate (PIP3) and modulates insulin signaling. Biochem Bioph Res Co 2009; 390: 1023–1028.
Wang W, Lee SB, Palmer R, Eillisen LW, Haber DA . A functional interaction with CBP contributes to transcriptional activator by the Wilms’ tumor suppressor WT1. J Biol Chem 2001; 276: 16810–16816.
Morrow IC, Parton RG . Flotillins and the PHB domain protein family: rafts, worms and anaesthetics. Traffic 2005; 6: 725–740.
Gozani O, Karuman P, Jones DR, Ivanov D, Cha J, Lugovskoy AA et al. The PHD finger of the chromatin-associated protein ING2 functions as a nuclear phosphoinositide receptor. Cell 2003; 114: 99–111.
Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK et al. Regulation of histone acetylation in the nucleus by sphingosine 1-phosphate. Science 2009; 325: 1254–1257.
Han BK, Emr SD . Phosphoinositide [PI (3,5)P2] lipid-dependent regulation of the general transcriptional regulator Tup1. Genes Dev 2011; 25: 984–995.
Zhao K, Wang W, Rando OJ, Xue Y, Swiderek K, Kuo A et al. Rapid and phosphoinositol-dependent binding of the SWI-SNF-like BAF complex to chromatin after T lumphocyte receptor signaling. Cell 1998; 95: 625–636.
Steger DJ, Haswell ES, Miller AL, Wente SR, O’Shea EK . Regulation of chromatin remodeling by inositol polyphosphates. Science 2003; 299: 114–116.
Watson PJ, Fairall L, Santos GM . Schwabe JWR. structure of HDAC3 bound to co-repressor and inositol tetraphosphate. Nature 2012; 481: 335–340.
Millard CJ, Watson PJ, Celardo I, Gordiyenko Y, Cowley SM, Robinson CV et al. Class I HDACs share a common mechanism of regulation by inositol phosphates. Mol Cell 2013; 51: 1–11.
Yildirim S, Castano E, Sobol M, Philimonenko VV, Dzijak R, Venit T et al. Involvement of phosphatidylinositol 4,5-bisphosphate in RNA polymerase 1 transcription. J Cell Sci 2013; 126: 2730–2739.
He B, Kim TH, Kommagani R, Feng Q, Lanz RB, Jeong JW et al. Estrogen-regulated prohibitin is required for mouse uterine development and adult function. Endrocrinology 2012; 152: 1047.
Shandilya J, Wang Y, Roberts SGE . TFIIB dephosphorylation links transcription inhibition with the p53-dependent DNA damage response. Proc Natl Acad Sci USA 2012; 10: 1073.
Acknowledgements
This work was funded by the National Institute of General Medical Sciences (1R01GM098609) (to KFM and SGER) and Cancer Research UK (C1356/A6630) (to SGER). We thank Alan Siegel for help with the confocal microscopy and the facility funded by National Science Foundation (DBI 0923133).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Toska, E., Shandilya, J., Goodfellow, S. et al. Prohibitin is required for transcriptional repression by the WT1–BASP1 complex. Oncogene 33, 5100–5108 (2014). https://doi.org/10.1038/onc.2013.447
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.447
Keywords
This article is cited by
-
Prohibitin 1 interacts with p53 in the regulation of mitochondrial dynamics and chemoresistance in gynecologic cancers
Journal of Ovarian Research (2022)
-
Methylation-associated silencing of BASP1 contributes to leukemogenesis in t(8;21) acute myeloid leukemia
Experimental & Molecular Medicine (2018)
-
Significance of prohibitin domain family in tumorigenesis and its implication in cancer diagnosis and treatment
Cell Death & Disease (2018)
-
IDPpi: Protein-Protein Interaction Analyses of Human Intrinsically Disordered Proteins
Scientific Reports (2018)
-
High brain acid soluble protein 1(BASP1) is a poor prognostic factor for cervical cancer and promotes tumor growth
Cancer Cell International (2017)