Abstract
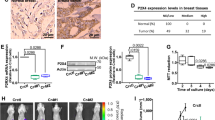
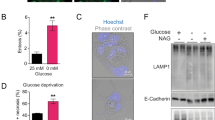
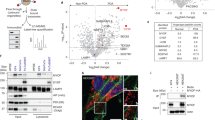
Haeme-responsive gene (HRG)-1 encodes a 16-kDa transmembrane protein that is induced by insulin-like growth factor-1 (IGF-1) and associates with the vacuolar-(H+) ATPase (V-ATPase). We previously reported that HRG-1 is essential for V-ATPase activity in endosomal acidification and receptor trafficking. Here, we show that in highly invasive and migratory cancer cell lines, HRG-1 and the V-ATPase are co-expressed at the plasma membrane, whereas in less invasive cell lines and non-transformed cells HRG-1 over-expression remains confined to intracellular compartments. Stable suppression of HRG-1 in invasive breast cancer MDA-MB-231 cells decreases extracellular pH, cell growth, migration and invasion. Ectopic expression of HRG-1 in non-invasive MCF-7 cells enhances V-ATPase activity, lowers the extracellular pH and increases the pH-dependent activity of MMP2 and MMP9 matrix metalloproteinases. HRG-1 enhances trafficking of the glucose transporter-1 (GLUT-1) with a concomitant increase in glucose uptake and lactate production. HRG-1 also promotes trafficking of the insulin-like growth factor I receptor (IGF-1R), β1-integrin and IGF-1 signalling. Taken together, our findings indicate that HRG-1 expression at the plasma membrane enhances V-ATPase activity, drives glycolytic flux and facilitates cancer cell growth, migration and invasion. Thus, HRG-1 may represent a novel target for selectively disrupting V-ATPase activity and the metastatic potential of cancer cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Webb BA, Chimenti M, Jacobson MP, Barber DL . Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer 2011; 11: 671–677.
Cardone Ra Casavola V, Reshkin SJ . The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer 2005; 5: 786–795.
Swietach P, Hulikova A, Vaughan-Jones R, Harris A . New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation. Oncogene 2010; 29: 6509–6521.
Chiche J, Fur YL, Vilmen C, Frassineti F, Daniel L, Halestrap AP et al. In vivo pH in metabolic‐defective Ras‐transformed fibroblast tumors: Key role of the monocarboxylate transporter MCT4, for inducing an alkaline intracellular pH. Int J Cancer 2011; 130: 1511–1520.
Hernandez A, Serrano-Bueno G, Perez-Castineira RJ, Serrano A . Intracellular proton pumps as targets in chemotherapy: V-ATPases and cancer. Curr Pharm Des 2012; 18: 1383–1394.
Toei M, Saum R, Forgac M . Regulation and isoform function of the V-ATPases. Biochemistry 2010; 49: 4715–4723.
Forgac M . Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 2007; 8: 917–929.
Wagner Ca Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP . Renal vacuolar H+-ATPase. Physiol Rev 2004; 84: 1263–1314.
Toyomura T, Murata Y, Yamamoto A, Oka T, Sun-Wada G-H, Wada Y et al. From lysosomes to the plasma membrane: localization of vacuolar-type H+ -ATPase with the a3 isoform during osteoclast differentiation. J Biol Chem 2003; 278: 22023–22030.
Pastor-Soler N, Piétrement C, Breton S . Role of acid/base transporters in the male reproductive tract and potential consequences of their malfunction. Physiol 2005; 20: 417–428.
Hinton A, Sennoune SR, Bond S, Fang M, Reuveni M, Sahagian GG et al. Function of a subunit isoforms of the V-ATPase in pH homeostasis and in vitro invasion of MDA-MB231 human breast cancer cells. J Biol Chem 2009; 284: 16400–16408.
Martinez-Zaguilan R, Lynch RM, Martinez GM, Gillies RJ, Vacuolar-type H . (+)-ATPases are functionally expressed in plasma membranes of human tumor cells. Am J Physiol Cell Physiol 1993; 265: C1015–C1029.
Sennoune SR, Bakunts K, Martínez GM, Chua-Tuan JL, Kebir Y, Attaya MN et al. Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am J Physiol Cell Physiol 2004; 286: C1443–C1452.
Martínez-Zaguilán R, Raghunand N, Lynch RM, Bellamy W, Martinez GM, Rojas B et al. pH and drug resistance. I Functional expression of plasmalemmal V-type H+-ATPase in drug-resistant human breast carcinoma cell lines. Biochem Pharmacol 1999; 57: 1037–1046.
Nishisho T, Hata K, Nakanishi M, Morita Y, Sun-Wada GH, Wada Y et al. The a3 isoform vacuolar type H+-ATPase promotes distant metastasis in the mouse B16 melanoma cells. Mol Cancer Res 2011; 9: 845–855.
Pérez-Sayáns M, Suárez-Peñaranda J, Barros-Angueira F, Diz P, Gándara-Rey J, García-García A . An update in the structure, function, and regulation of V-ATPases: the role of the C subunit. Braz J Biol 2012; 72: 189–198.
Parra KJ, Kane PM . Reversible association between the V1and V0 domains of yeast vacuolar H+-ATPase is an unconventional glucose-induced effect. Mol Cell Biol 1998; 18: 7064–7074.
Beyenbach KW, Wieczorek H . The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J Exper Biol 2006; 209: 577–589.
Sautin YY, Lu M, Gaugler A, Zhang L, Gluck SL . Phosphatidylinositol 3-kinase-mediated effects of glucose on vacuolar H+-ATPase assembly, translocation, and acidification of intracellular compartments in renal epithelial cells. Mol Cell Biol 2005; 25: 575–589.
Su Y, Zhou A, Al-Lamki RS, Karet FE . The a-subunit of the V-type H+-ATPase interacts with phosphofructokinase-1 in humans. J Biol Chem 2003; 278: 20013–20018.
Lu M . The glycolytic enzyme aldolase mediates assembly, expression, and activity of vacuolar H+-ATPase. J Biol Chem 2004; 279: 8732–8739.
O’Callaghan KM, Ayllon V, O'Keeffe J, Wang Y, Cox OT, Loughran G et al. Heme-binding protein HRG-1 is induced by insulin-like growth factor I and associates with the vacuolar H+-ATPase to control endosomal pH and receptor trafficking. J Biol Chem 2010; 285: 381–391.
Thomsen P, Rudenko O, Berezin V, Norrild B . The HPV-16 E5 oncogene and bafilomycin A(1) influence cell motility. Biochim Biophys Acta 1999; 1452: 285–295.
Spugnini EP, Citro G, Fais S . Proton pump inhibitors as anti vacuolar-ATPases drugs: a novel anticancer strategy. J Exper Clin Cancer Res 2010; 29: 44.
Kubota S, Seyama Y . Overexpression of vacuolar ATPase 16-kDa subunit in 10T1/2 fibroblasts enhances invasion with concomitant induction of matrix metalloproteinase-2. Biochem Biophys Res Commun 2000; 278: 390–394.
Dechant R, Binda M, Lee SS, Pelet S, Winderickx J, Peter M . Cytosolic pH is a second messenger for glucose and regulates the PKA pathway through V-ATPase. EMBO J 2010; 29: 2515–2526.
Clarke JF, Young P, Yonezawa K, Kasuga M, Holman G . Inhibition of the translocation of GLUT1 and GLUT4 in 3T3-L1 cells by the phosphatidylinositol 3-kinase inhibitor, wortmannin. Biochem J 1994; 300 (Pt 3): 631.
Samih N, Hovsepian S, Aouani A, Lombardo D, Fayet G . Glut-1 translocation in FRTL-5 thyroid cells: role of phosphatidylinositol 3-kinase and N-glycosylation. Endocrinology 2000; 141: 4146–4155.
Martínez-Muñoz Ga Kane P . Vacuolar and plasma membrane proton pumps collaborate to achieve cytosolic pH homeostasis in yeast. J Biol Chem 2008; 283: 20309–20319.
Yanatori I, Tabuchi M, Kawai Y, Yasui Y, Akagi R, Kishi F . Heme and non-heme iron transporters in non-polarized and polarized cells. BMC Cell Biol 2010; 11: 39.
Balgi AD, Diering GH, Donohue E, Lam KKY, Fonseca BD, Zimmerman C et al. Regulation of mTORC1 signaling by pH. PLoS One 2011; 6: e21549.
Murakami T, Shibuya I, Ise T, Chen ZS, Akiyama S, Nakagawa M et al. Elevated expression of vacuolar proton pump genes and cellular PH in cisplatin resistance. Int J Cancer 2001; 93: 869–874.
Ohta T, Arakawa H, Futagami F, Fushida S, Kitagawa H, Kayahara M et al. Bafilomycin A1 induces apoptosis in the human pancreatic cancer cell line Capan‐1. J Pathol 1999; 185: 324–330.
Perez-Sayans M, Somoza-Martin JM, Barros-Angueira F, Rey JMG, Garcia-Garcia A . V-ATPase inhibitors and implication in cancer treatment. Cancer Treat Rev 2009; 35: 707–713.
Sennoune RS, Martinez-Zaguilan R . Vacuolar H-ATPase signaling pathway in cancer. Curr Protein Pept Sci 2012; 13: 152–163.
Vander Heiden MG, Cantley LC, Thompson CB . Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324: 1029–1033.
Lu X, Qin W, Li J, Tan N, Pan D, Zhang H et al. The growth and metastasis of human hepatocellular carcinoma xenografts are inhibited by small interfering RNA targeting to the subunit ATP6L of proton pump. Cancer Res 2005; 65: 6843–6849.
Nilsson C, Kågedal K, Johansson U, Ollinger K . Analysis of cytosolic and lysosomal pH in apoptotic cells by flow cytometry. Methods Cell Sci 2003; 25: 185–194.
Zhdanov AV, Dmitriev RI, Golubeva AV, Gavrilova SA, Papkovsky DB . Chronic hypoxia leads to a glycolytic phenotype and suppressed HIF-2 signaling in PC12 cells. Biochim Biophys Acta 2013; 1830: 3553–3569.
Acknowledgements
We are grateful to our colleagues in the Cell Biology Laboratory and Dr Kellie Dean for helpful discussions, and to Kurt Tidmore for preparing illustrations. This work was supported by the Health Research Board (PhD Scholars Programme in Cancer Biology (FF)) and Science Foundation Ireland (PI Award: 06/INI/B107).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Fogarty, F., O'Keeffe, J., Zhadanov, A. et al. HRG-1 enhances cancer cell invasive potential and couples glucose metabolism to cytosolic/extracellular pH gradient regulation by the vacuolar-H+ ATPase. Oncogene 33, 4653–4663 (2014). https://doi.org/10.1038/onc.2013.403
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.403
Keywords
This article is cited by
-
Nutrient transporters: connecting cancer metabolism to therapeutic opportunities
Oncogene (2023)
-
The V-ATPases in cancer and cell death
Cancer Gene Therapy (2022)
-
TM9SF4 is a novel V-ATPase-interacting protein that modulates tumor pH alterations associated with drug resistance and invasiveness of colon cancer cells
Oncogene (2015)