Abstract
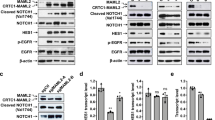
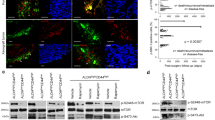
Salivary gland tumors (SGT) are a group of highly heterogeneous head and neck malignancies with widely varied clinical outcomes and no standard effective treatments. The CRTC1–MAML2 fusion oncogene, encoded by a recurring chromosomal translocation t(11;19)(q14-21;p12-13), is a frequent genetic alteration found in >50% of mucoepidermoid carcinomas (MEC), the most common malignant SGT. In this study, we aimed to define the role of the CRTC1–MAML2 oncogene in the maintenance of MEC tumor growth and to investigate critical downstream target genes and pathways for therapeutic targeting of MEC. By performing gene expression analyses and functional studies via RNA interference and pharmacological modulation, we determined the importance of the CRTC1–MAML2 fusion gene and its downstream AREG–EGFR signaling in human MEC cancer cell growth and survival in vitro and in vivo using human MEC xenograft models. We found that CRTC1–MAML2 fusion oncogene was required for the growth and survival of fusion-positive human MEC cancer cells in vitro and in vivo. The CRTC1–MAML2 oncoprotein induced the upregulation of the epidermal growth factor receptor (EGFR) ligand Amphiregulin (AREG) by co-activating the transcription factor CREB, and AREG subsequently activated EGFR signaling in an autocrine manner that promoted MEC cell growth and survival. Importantly, CRTC1–MAML2-positive MEC cells were highly sensitive to EGFR signaling inhibition. Therefore, our study revealed that aberrantly activated AREG–EGFR signaling is required for CRTC1–MAML2-positive MEC cell growth and survival, suggesting that EGFR-targeted therapies will benefit patients with advanced, unresectable CRTC1–MAML2-positive MEC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Eveson JW . Salivary tumours. Periodontol 2000 2011; 57: 150–159.
Bell D, Hanna EY . Salivary gland cancers: biology and molecular targets for therapy. Curr Oncol Rep 2012; 14: 166–174.
Stenman G . Fusion oncogenes and tumor type specificity—insights from salivary gland tumors. Semin Cancer Biol 2005; 15: 224–235.
Persson M, Andren Y, Mark J, Horlings HM, Persson F, Stenman G . Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci USA 2009; 106: 18740–18744.
O’Neill ID . New insights into the nature of Warthin’s tumour. J Oral Pathol Med 2009; 38: 145–149.
O’Neill ID . t(11;19) translocation and CRTC1-MAML2 fusion oncogene in mucoepidermoid carcinoma. Oral Oncol 2009; 45: 2–9.
Behboudi A, Enlund F, Winnes M, Andren Y, Nordkvist A, Leivo I et al. Molecular classification of mucoepidermoid carcinomas-prognostic significance of the MECT1-MAML2 fusion oncogene. Genes Chromosomes Cancer 2006; 45: 470–481.
Enlund F, Behboudi A, Andren Y, Oberg C, Lendahl U, Mark J et al. Altered Notch signaling resulting from expression of a WAMTP1-MAML2 gene fusion in mucoepidermoid carcinomas and benign Warthin’s tumors. Exp Cell Res 2004; 292: 21–28.
Tonon G, Modi S, Wu L, Kubo A, Coxon AB, Komiya T et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet 2003; 33: 208–213.
Kumar V, Abbas A, Fausto N . Robbins & Cotran Pathologic Basis of Disease 7th edn Elsevier, Saunders, 2005.
Seethala RR, Dacic S, Cieply K, Kelly LM, Nikiforova MN . A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol 2010; 34: 1106–1121.
Chiosea SI, Dacic S, Nikiforova MN, Seethala RR . Prospective testing of mucoepidermoid carcinoma for the MAML2 translocation: clinical implications. Laryngoscope 2012; 122: 1690–1694.
Fehr A, Roser K, Belge G, Loning T, Bullerdiek J . A closer look at Warthin tumors and the t(11;19). Cancer Genet Cytogenet 2008; 180: 135–139.
Wu L, Liu J, Gao P, Nakamura M, Cao Y, Shen H et al. Transforming activity of MECT1-MAML2 fusion oncoprotein is mediated by constitutive CREB activation. EMBO J 2005; 24: 2391–2402.
Iourgenko V, Zhang W, Mickanin C, Daly I, Jiang C, Hexham JM et al. Identification of a family of cAMP response element-binding protein coactivators by genome-scale functional analysis in mammalian cells. Proc Natl Acad Sci USA 2003; 100: 12147–12152.
Conkright MD, Canettieri G, Screaton R, Guzman E, Miraglia L, Hogenesch JB et al. TORCs: transducers of regulated CREB activity. Mol Cell 2003; 12: 413–423.
Komiya T, Coxon A, Park Y, Chen WD, Zajac-Kaye M, Meltzer P et al. Enhanced activity of the CREB co-activator Crtc1 in LKB1 null lung cancer. Oncogene 2010; 29: 1672–1680.
Altarejos JY, Montminy M . CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol 2011; 12: 141–151.
Gu Y, Lin S, Li JL, Nakagawa H, Chen Z, Jin B et al. Altered LKB1/CREB-regulated transcription co-activator (CRTC) signaling axis promotes esophageal cancer cell migration and invasion. Oncogene 2012; 31: 469–479.
Wu L, Sun T, Kobayashi K, Gao P, Griffin JD . Identification of a family of mastermind-like transcriptional coactivators for mammalian notch receptors. Mol Cell Biol 2002; 22: 7688–7700.
McElhinny AS, Li JL, Wu L . Mastermind-like transcriptional co-activators: emerging roles in regulating cross talk among multiple signaling pathways. Oncogene 2008; 27: 5138–5147.
Coxon A, Rozenblum E, Park YS, Joshi N, Tsurutani J, Dennis PA et al. Mect1-Maml2 fusion oncogene linked to the aberrant activation of cyclic AMP/CREB regulated genes. Cancer Res 2005; 65: 7137–7144.
Komiya T, Park Y, Modi S, Coxon AB, Oh H, Kaye FJ . Sustained expression of Mect1-Maml2 is essential for tumor cell growth in salivary gland cancers carrying the t(11;19) translocation. Oncogene 2006; 25: 6128–6132.
Canettieri G, Coni S, Della Guardia M, Nocerino V, Antonucci L, Di Magno L et al. The coactivator CRTC1 promotes cell proliferation and transformation via AP-1. Proc Natl Acad Sci USA 2009; 106: 1445–1450.
Busser B, Sancey L, Brambilla E, Coll JL, Hurbin A . The multiple roles of amphiregulin in human cancer. Biochim Biophys Acta 2011; 1816: 119–131.
Grisanti S, Amoroso V, Buglione M, Rosati A, Gatta R, Pizzocaro C et al. Cetuximab in the treatment of metastatic mucoepidermoid carcinoma of the salivary glands: a case report and review of literature. J Med Case Reports 2008; 2: 320.
Han SW, Kim HP, Jeon YK, Oh DY, Lee SH, Kim DW et al. Mucoepidermoid carcinoma of lung: potential target of EGFR-directed treatment. Lung Cancer 2008; 61: 30–34.
O’Neill ID . Gefitinib as targeted therapy for mucoepidermoid carcinoma of the lung: possible significance of CRTC1-MAML2 oncogene. Lung Cancer 2009; 64: 129–130.
Lujan B, Hakim S, Moyano S, Nadal A, Caballero M, Diaz A et al. Activation of the EGFR/ERK pathway in high-grade mucoepidermoid carcinomas of the salivary glands. Br J Cancer 2010; 103: 510–516.
Anzick SL, Chen WD, Park Y, Meltzer P, Bell D, El-Naggar AK et al. Unfavorable prognosis of CRTC1-MAML2 positive mucoepidermoid tumors with CDKN2A deletions. Genes Chromosomes Cancer 2010; 49: 59–69.
Yonesaka K, Zejnullahu K, Lindeman N, Homes AJ, Jackman DM, Zhao F et al. Autocrine production of amphiregulin predicts sensitivity to both gefitinib and cetuximab in EGFR wild-type cancers. Clin Cancer Res 2008; 14: 6963–6973.
Raben D, Helfrich B, Chan DC, Ciardiello F, Zhao L, Franklin W et al. The effects of cetuximab alone and in combination with radiation and/or chemotherapy in lung cancer. Clin Cancer Res 2005; 11: 795–805.
Steiner P, Joynes C, Bassi R, Wang S, Tonra JR, Hadari YR et al. Tumor growth inhibition with cetuximab and chemotherapy in non-small cell lung cancer xenografts expressing wild-type and mutated epidermal growth factor receptor. Clin Cancer Res 2007; 13: 1540–1551.
Wu L, Aster JC, Blacklow SC, Lake R, Artavanis-Tsakonas S, Griffin JD . MAML1, a human homologue of Drosophila mastermind, is a transcriptional co-activator for NOTCH receptors. Nat Genet 2000; 26: 484–489.
Acknowledgements
We thank Marda Jorgensen for helping with immunohistochemical staining, Mei Zhang for helping with FACS analysis, and the Salivary Gland Tumor Biorepository (SGTB) for providing tumor specimens for this study. L Wu was supported by the University of Florida Shands Cancer Center Startup fund, Bankhead Coley Cancer Research Program, and the V foundation for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, Z., Chen, J., Gu, Y. et al. Aberrantly activated AREG–EGFR signaling is required for the growth and survival of CRTC1–MAML2 fusion-positive mucoepidermoid carcinoma cells. Oncogene 33, 3869–3877 (2014). https://doi.org/10.1038/onc.2013.348
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.348
Keywords
This article is cited by
-
The evolving role of molecular pathology in the diagnosis of salivary gland tumours with potential pitfalls
European Archives of Oto-Rhino-Laryngology (2022)
-
Targeting Notch and EGFR signaling in human mucoepidermoid carcinoma
Signal Transduction and Targeted Therapy (2021)
-
Histologie und Molekularpathologie von Speicheldrüsentumoren
Der MKG-Chirurg (2021)
-
Functional linkage of gene fusions to cancer cell fitness assessed by pharmacological and CRISPR-Cas9 screening
Nature Communications (2019)
-
The rationale for druggability of CCDC6-tyrosine kinase fusions in lung cancer
Molecular Cancer (2018)