Abstract
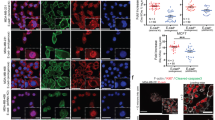
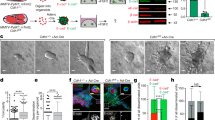
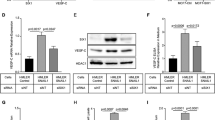
N-cadherin and HER2/neu were found to be co-expressed in invasive breast carcinomas. To test the contribution of N-cadherin and HER2 in mammary tumor metastasis, we targeted N-cadherin expression in the mammary epithelium of the MMTV-Neu mouse. In the context of ErbB2/Neu, N-cadherin stimulated carcinoma cell invasion, proliferation and metastasis. N-cadherin caused fibroblast growth factor receptor (FGFR) upmodulation, resulting in epithelial-to-mesenchymal transition (EMT) and stem/progenitor like properties, involving Snail and Slug upregulation, mammosphere formation and aldehyde dehydrogenase activity. N-cadherin potentiation of the FGFR stimulated extracellular signal regulated kinase (ERK) and protein kinase B (AKT) phosphorylation resulting in differential effects on metastasis. Although ERK inhibition suppressed cyclin D1 expression, cell proliferation and stem/progenitor cell properties, it did not affect invasion or EMT. Conversely, AKT inhibition suppressed invasion through Akt 2 attenuation, and EMT through Snail inhibition, but had no effect on cyclin D1 expression, cell proliferation or mammosphere formation. These findings suggest N-cadherin/FGFR has a pivotal role in promoting metastasis through differential regulation of ERK and AKT, and underscore the potential for targeting the FGFR in advanced ErbB2-amplified breast tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Thiery JP, Acloque H, Huang RY, Nieto MA . Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139: 871–890.
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL . Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235: 177–182.
Eccles SA . The role of c-erbB-2/HER2/neu in breast cancer progression and metastasis. J Mammary Gland Biol Neoplasia 2001; 6: 393–406.
Suyama K, Shapiro I, Guttman M, Hazan RB . A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell 2002; 2: 301–314.
Hulit J, Suyama K, Chung S, Keren R, Agiostratidou G, Shan W et al. N-cadherin signaling potentiates mammary tumor metastasis via enhanced extracellular signal-regulated kinase activation. Cancer Res 2007; 67: 3106–3116.
Nagi C, Guttman M, Jaffer S, Qiao R, Keren R, Triana A et al. N-cadherin expression in breast cancer: correlation with an aggressive histologic variant—invasive micropapillary carcinoma. Breast Cancer Res Treat 2005; 94: 225–235.
Walsh MM, Bleiweiss IJ . Invasive micropapillary carcinoma of the breast: eighty cases of an underrecognized entity. Hum Pathol 2001; 32: 583–589.
Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P . Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell 1988; 54: 105–115.
Bargmann CI, Hung MC, Weinberg RA . Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell 1986; 45: 649–657.
Knudsen KA, Sauer C, Johnson KR, Wheelock MJ . Effect of N-cadherin misexpression by the mammary epithelium in mice. J Cell Biochem 2005; 95: 1093–1107.
Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One 2009; 4: e6562.
Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA . Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol 2000; 148: 779–790.
Larocca D, Witte A, Gonzalez AM, Houston LL . Establishment of epitope-defined monoclonal antibodies with specificity for fibroblast growth factor receptor types 1 and 2. Hybridoma 1998; 17: 21–31.
Mohammadi M, Froum S, Hamby JM, Schroeder MC, Panek RL, Lu GH et al. Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. EMBO J 1998; 17: 5896–5904.
Kouhara H, Hadari YR, Spivak-Kroizman T, Schilling J, Bar-Sagi D, Lax I et al. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 1997; 89: 693–702.
Tomaskovic-Crook E, Thompson EW, Thiery JP . Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res 2009; 11: 213.
Chin YR, Toker A . Function of Akt/PKB signaling to cell motility, invasion and the tumor stroma in cancer. Cell Signal 2009; 21: 470–476.
Yoeli-Lerner M, Yiu GK, Rabinovitz I, Erhardt P, Jauliac S, Toker A . Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol Cell 2005; 20: 539–550.
Xue G, Hemmings BA . PKB/Akt-dependent regulation of cell motility. J Natl Cancer Inst 2013; 105: 393–404.
Chung S, Yao J, Suyama K, Bajaj S, Qian X, Loudig OD et al. N-cadherin regulates mammary tumor cell migration through Akt3 suppression. Oncogene 2013; 32: 422–430.
Toker A, Yoeli-Lerner M . Akt signaling and cancer: surviving but not moving on. Cancer Res 2006; 66: 3963–3966.
Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N et al. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol 2005; 171: 1023–1034.
Debnath J, Walker SJ, Brugge JS . Akt activation disrupts mammary acinar architecture and enhances proliferation in an mTOR-dependent manner. J Cell Biol 2003; 163: 315–326.
Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH . Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res 2007; 67: 1979–1987.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133: 704–715.
Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS . Stem cells in normal breast development and breast cancer. Cell Prolif 2003; 36 (Suppl 1): 59–72.
Liao MJ, Zhang CC, Zhou B, Zimonjic DB, Mani SA, Kaba M et al. Enrichment of a population of mammary gland cells that form mammospheres and have in vivo repopulating activity. Cancer Res 2007; 67: 8131–8138.
Gotoh N . Control of stemness by fibroblast growth factor signaling in stem cells and cancer stem cells. Curr Stem Cell Res Ther 2009; 4: 9–15.
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007; 1: 555–567.
Lim E, Wu D, Pal B, Bouras T, Asselin-Labat ML, Vaillant F et al. Transcriptome analyses of mouse and human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast Cancer Res 2010; 12: R21.
Eirew P, Kannan N, Knapp DJ, Vaillant F, Emerman JT, Lindeman GJ et al. Aldehyde dehydrogenase activity is a biomarker of primitive normal human mammary luminal cells. Stem Cells 2012; 30: 344–348.
Korkaya H, Paulson A, Iovino F, Wicha MS . HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene 2008; 27: 6120–6130.
Nahta R, Esteva FJ . HER2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res 2006; 8: 215.
Giordano A, Gao H, Anfossi S, Cohen E, Mego M, Lee BN et al. Epithelial-mesenchymal transition and stem cell markers in patients with HER2-positive metastatic breast cancer. Mol Cancer Ther 2012; 11: 2526–2534.
Warzecha CC, Sato TK, Nabet B, Hogenesch JB, Carstens RP . ESRP1 and ESRP2 are epithelial cell-type-specific regulators of FGFR2 splicing. Mol Cell 2009; 33: 591–601.
Warzecha CC, Shen S, Xing Y, Carstens RP . The epithelial splicing factors ESRP1 and ESRP2 positively and negatively regulate diverse types of alternative splicing events. RNA Biol 2009; 6: 546–562.
Shapiro IM, Cheng AW, Flytzanis NC, Balsamo M, Condeelis JS, Oktay MH et al. An EMT-driven alternative splicing program occurs in human breast cancer and modulates cellular phenotype. PLoS Genet 7: e1002218.
Ocana OH, Corcoles R, Fabra A, Moreno-Bueno G, Acloque H, Vega S et al. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell 2012; 22: 709–724.
Qian X, Hulit J, Suyama K, Eugenin EA, Belbin TJ, Loudig O et al. p21CIP1 mediates reciprocal switching between proliferation and invasion during metastasis. Oncogene 2012; 32: 2292–2303.
Lelievre EC, Plestant C, Boscher C, Wolff E, Mege RM, Birbes H . N-cadherin mediates neuronal cell survival through Bim down-regulation. PLoS One 2012; 7: e33206.
Visvader JE, Lindeman GJ . Cancer stem cells: current status and evolving complexities. Cell Stem Cell 2012; 10: 717–728.
Smalley MJ, Kendrick H, Sheridan JM, Regan JL, Prater MD, Lindeman GJ et al. Isolation of mouse mammary epithelial subpopulations: a comparison of leading methods. J Mammary Gland Biol Neoplasia 2012; 17: 91–97.
Koziczak M, Holbro T, Hynes NE . Blocking of FGFR signaling inhibits breast cancer cell proliferation through downregulation of D-type cyclins. Oncogene 2004; 23: 3501–3508.
Koziczak M, Hynes NE . Cooperation between fibroblast growth factor receptor-4 and ErbB2 in regulation of cyclin D1 translation. J Biol Chem 2004; 279: 50004–50011.
Issa A, Gill JW, Heideman MR, Sahin O, Wiemann S, Dey JH et al. Combinatorial targeting of FGF and ErbB receptors blocks growth and metastatic spread of breast cancer models. Breast Cancer Res 2013; 15: R8.
Sharpe R, Pearson A, Herrera-Abreu MT, Johnson D, Mackay A, Welti JC et al. FGFR signaling promotes the growth of triple-negative and basal-like breast cancer cell lines both in vitro and in vivo. Clin Cancer Res 2011; 17: 5275–5286.
Acknowledgements
We thank Dr Jeffrey Segall for providing the wild-type MMTV-ErbB2 mouse. We thank Dr Peng Guo for expert assistance in confocal imaging and the imaging facility at Albert Einstein College of Medicine. This work was supported by grants from the Breast Cancer Research Foundation (RB Hazan and Larry Norton) and the National Cancer Institute grant (1R01 CA135061-01A1; RB Hazan). This publication was also supported in part by the CTSA Grant UL1RR025750, KL2RR025749 and TL1RR025748 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH roadmap for Medical Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Qian, X., Anzovino, A., Kim, S. et al. N-cadherin/FGFR promotes metastasis through epithelial-to-mesenchymal transition and stem/progenitor cell-like properties. Oncogene 33, 3411–3421 (2014). https://doi.org/10.1038/onc.2013.310
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.310
Keywords
This article is cited by
-
Unleashing the potential of combining FGFR inhibitor and immune checkpoint blockade for FGF/FGFR signaling in tumor microenvironment
Molecular Cancer (2023)
-
Epigenetic targeting of neuropilin-1 prevents bypass signaling in drug-resistant breast cancer
Oncogene (2021)
-
Glycosphingolipid expression at breast cancer stem cells after novel thieno[2,3-b]pyridine anticancer compound treatment
Scientific Reports (2020)
-
Shisa3 brakes resistance to EGFR-TKIs in lung adenocarcinoma by suppressing cancer stem cell properties
Journal of Experimental & Clinical Cancer Research (2019)
-
N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer
BMC Cancer (2018)