Abstract
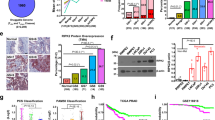
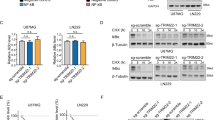
Nuclear factor-κB (NF-κB) signaling contributes to human disease processes, notably inflammatory diseases and cancer. NF-κB has a role in tumorigenesis and tumor growth, as well as promotion of metastases. Mechanisms responsible for abnormal NF-κB activation are not fully elucidated; however, RelA phosphorylation, particularly at serine residues S536 and S276, is critical for RelA function. Kinases that phosphorylate RelA promote oncogenic behaviors, suggesting that phosphatases targeting RelA could have tumor-inhibiting activities; however, few RelA phosphatases have been identified. Here, we identified tumor inhibitory and RelA phosphatase activities of the protein phosphatase 2C (PP2C) phosphatase family member, PPM1A. We show that PPM1A directly dephosphorylated RelA at residues S536 and S276 and selectively inhibited NF-κB transcriptional activity, resulting in decreased expression of monocyte chemotactic protein-1/chemokine (C–C motif) ligand 2 and interleukin-6, cytokines implicated in cancer metastasis. PPM1A depletion enhanced NF-κB-dependent cell invasion, whereas PPM1A expression inhibited invasion. Analyses of human expression data revealed that metastatic prostate cancer deposits had lower PPM1A expression compared with primary tumors without distant metastases. A hematogenous metastasis mouse model revealed that PPM1A expression inhibited bony metastases of prostate cancer cells after vascular injection. In summary, our findings suggest that PPM1A is a RelA phosphatase that regulates NF-κB activity and that PPM1A has tumor suppressor-like activity. Our analyses also suggest that PPM1A inhibits prostate cancer metastases and as neither gene deletions nor inactivating mutations of PPM1A have been described, increasing PPM1A activity in tumors represents a potential therapeutic strategy to inhibit NF-κB signaling or bony metastases in human cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Grivennikov SI, Karin M . Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev 2010; 20: 65–71.
Orlowski RZ, Baldwin J, Albert S . NF-[kappa]B as a therapeutic target in cancer. Trends Mol Med 2002; 8: 385–389.
Wang J, An H, Mayo MW, Baldwin AS, WG Yarbrough . LZAP a putative tumor suppressor, selectively inhibits NF-kappaB. Cancer Cell 2007; 12: 239–251.
Arun P, Brown MS, Ehsanian R, Chen Z, Van Waes C . Nuclear NF-kappaB p65 phosphorylation at serine 276 by protein kinase A contributes to the malignant phenotype of head and neck cancer. Clin Cancer Res 2009; 15: 5974–5984.
Cao Y, Karin M . NF-kappaB in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia 2003; 8: 215–223.
Wu JT, Kral JG . The NF-kappaB/IkappaB signaling system: a molecular target in breast cancer therapy. J Surg Res 2005; 123: 158–169.
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ . Cancer statistics, 2007. CA Cancer J Clin 2007; 57: 43–66.
Nelson WG, De Marzo AM, Isaacs WB . Prostate cancer. N Engl J Med 2003; 349: 366–381.
Min J, Zaslavsky A, Fedele G, McLaughlin SK, Reczek EE, De Raedt T et al. An oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-kappaB. Nat Med 2010; 16: 286–294.
Zhang J, Patel L, Pienta KJ . Targeting chemokine (C–C motif) ligand 2 (CCL2) as an example of translation of cancer molecular biology to the clinic. Prog Mol Biol Transl Sci 2010; 95: 31–53.
Craig MJ, Loberg RD . CCL2 (Monocyte Chemoattractant Protein-1) in cancer bone metastases. Cancer Metastasis Rev 2006; 25: 611–619.
Chen L-F, Green WC . Shaping the nuclear action of NF-κB. Nat Rev Mol Cell Biol 2004; 5: 392–401.
Neumann M, Naumann M . Beyond IkappaBs: alternative regulation of NF-kappaB activity. FASEB J 2007; 21: 2642–2654.
Sakurai H, Suzuki S, Kawasaki N, Nakano H, Okazaki T, Chino A et al. Tumor necrosis factor-alpha-induced IKK phosphorylation of NF-kappaB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J Biol Chem 2003; 278: 36916–36923.
Zhong H, May MJ, Jimi E, Ghosh S . The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell 2002; 9: 625–636.
Nelson DE, Ihekwaba AE, Elliott M, Johnson JR, Gibney CA, Foreman BE et al. Oscillations in NF-kappaB signaling control the dynamics of gene expression. Science 2004; 306: 704–708.
Yang J, Fan GH, Wadzinski BE, Sakurai H, Richmond A . Protein phosphatase 2A interacts with and directly dephosphorylates RelA. J Biol Chem 2001; 276: 47828–47833.
Chew J, Biswas S, Shreeram S, Humaidi M, Wong ET, Dhillion MK et al. WIP1 phosphatase is a negative regulator of NF-kappaB signalling. Nat Cell Biol 2009; 11: 659–666.
Lin X, Duan X, Liang YY, Su Y, Wrighton KH, Long J et al. PPM1A functions as a Smad phosphatase to terminate TGFbeta signaling. Cell 2006; 125: 915–928.
Lammers T, Lavi S . Role of type 2C protein phosphatases in growth regulation and in cellular stress signaling. Crit Rev Biochem Mol Biol 2007; 42: 437–461.
Shohat M, Ben-Meir D, Lavi S . Protein phosphatase magnesium dependent 1A (PPM1A) plays a role in the differentiation and survival processes of nerve cells. PLoS ONE 2012; 7: e32438.
Zhang B, Zhou Z, Lin H, Lv X, Fu J, Lin P et al. Protein phosphatase 1A (PPM1A) is involved in human cytotrophoblast cell invasion and migration. Histochem Cell Biol 2009; 132: 169–179.
Sun W, Yu Y, Dotti G, Shen T, Tan X, Savoldo B et al. PPM1A and PPM1B act as IKKbeta phosphatases to terminate TNFalpha-induced IKKbeta-NF-kappaB activation. Cell Signal 2009; 21: 95–102.
Buss H, Dorrie A, Schmitz ML, Frank R, Livingstone M, Resch K et al. Phosphorylation of serine 468 by GSK-3beta negatively regulates basal p65 NF-kappaB activity. J Biol Chem 2004; 279: 49571–49574.
Schwabe RF, Sakurai H . IKKbeta phosphorylates p65 at S468 in transactivaton domain 2. FASEB J 2005; 19: 1758–1760.
Perkins ND . Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene 2006; 25: 6717–6730.
Kim JS, Rho B, Lee TH, Lee JM, Kim SJ, Park JH . The interaction of hepatitis B virus X protein and protein phosphatase type 2 Calpha and its effect on IL-6. Biochem Biophys Res Commun 2006; 351: 253–258.
Ueda A, Okuda K, Ohno S, Shirai A, Igarashi T, Matsunaga K et al. NF-kappa B and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol 1994; 153: 2052–2063.
Ueda A, Ishigatsubo Y, Okubo T, Yoshimura T . Transcriptional regulation of the human monocyte chemoattractant protein-1 gene. Cooperation of two NF-kappaB sites and NF-kappaB/Rel subunit specificity. J Biol Chem 1997; 272: 31092–31099.
Ghosh S, Hayden MS . New regulators of NF-kappaB in inflammation. Nat Rev Immunol 2008; 8: 837–848.
Oeckinghaus A, Ghosh S . The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 2009; 1: a000034.
Wang CY, Mayo MW, Baldwin AS Jr . TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science 1996; 274: 784–787.
Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011; 475: 222–225.
Zhang J, Patel L, Pienta KJCC . chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev 2010; 21: 41–48.
Lu Y, Cai Z, Galson DL, Xiao G, Liu Y, George DE et al. Monocyte chemotactic protein-1 (MCP-1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate 2006; 66: 1311–1318.
Chandran UR, Ma C, Dhir R, Bisceglia M, Lyons-Weiler M, Liang W et al. Gene expression profiles of prostate cancer reveal involvement of multiple molecular pathways in the metastatic process. BMC Cancer 2007; 7: 64.
Yu YP, Landsittel D, Jing L, Nelson J, Ren B, Liu L et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol 2004; 22: 2790–2799.
Wu TT, Sikes RA, Cui Q, Thalmann GN, Kao C, Murphy CF et al. Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer 1998; 77: 887–894.
Ghosh S, Karin M . Missing pieces in the NF-kappaB puzzle. Cell 2002; 109 (Suppl 1): S81–S96.
Gardam S, Beyaert R . The kinase NIK as a therapeutic target in multiple myeloma. Expert Opin Ther Targets 2011; 15: 207–218.
Chaturvedi MM, Sung B, Yadav VR, Kannappan R, Aggarwal BB . NF-kappaB addiction and its role in cancer: 'one size does not fit all’. Oncogene 2011; 30: 1615–1630.
Yamaguchi H, Durell SR, Chatterjee DK, Anderson CW, Appella E . The Wip1 phosphatase PPM1D dephosphorylates SQ/TQ motifs in checkpoint substrates phosphorylated by PI3K-like kinases. Biochemistry 2007; 46: 12594–12603.
Yamaguchi H, Minopoli G, Demidov ON, Chatterjee DK, Anderson CW, Durell SR et al. Substrate specificity of the human protein phosphatase 2Cdelta, Wip1. Biochemistry 2005; 44: 5285–5294.
Browning DD, Pan ZK, Prossnitz ER, Ye RD . Cell type- and developmental stage-specific activation of NF-kappaB by fMet-Leu-Phe in myeloid cells. J Biol Chem 1997; 272: 7995–8001.
Smale ST . Hierarchies of NF-kappaB target-gene regulation. Nat Immunol 2011; 12: 689–694.
Azevedo A, Cunha V, Teixeira AL, Medeiros R . IL-6/IL-6R as a potential key signaling pathway in prostate cancer development. World J Clin Oncol 2011; 2: 384–396.
Wolf MJ, Hoos A, Bauer J, Boettcher S, Knust M, Weber A et al. Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2-Stat5 and p38MAPK pathway. Cancer Cell 2012; 22: 91–105.
Mueller L, Seggern LV, Schumacher J, Goumas F, Wilms C, Braun F et al. TNF-alpha similarly induces IL-6 and MCP-1 in fibroblasts from colorectal liver metastases and normal liver fibroblasts. Biochem Biophys Res Commun 2010; 397: 586–591.
Sosnoski DM, Krishnan V, Kraemer WJ, Dunn-Lewis C, Mastro AM . Changes in cytokines of the bone microenvironment during breast cancer metastasis. Int J Breast Cancer 2012; 2012: 160265.
Zollo M, Di Dato V, Spano D, De Martino D, Liguori L, Marino N et al. Targeting monocyte chemotactic protein-1 synthesis with bindarit induces tumor regression in prostate and breast cancer animal models. Clin Exp Metastasis 2012; 29: 585–601.
Coward J, Kulbe H, Chakravarty P, Leader D, Vassileva V, Leinster DA et al. Interleukin-6 as a therapeutic target in human ovarian cancer. Clin Cancer Res 2011; 17: 6083–6096.
Lammers T, Peschke P, Ehemann V, Debus J, Slobodin B, Lavi S et al. Role of PP2Calpha in cell growth, in radio- and chemosensitivity, and in tumorigenicity. Mol Cancer 2007; 6: 65.
Lu X, Ma O, Nguyen TA, Jones SN, Oren M, Donehower LA . The Wip1 phosphatase acts as a gatekeeper in the p53-Mdm2 autoregulatory loop. Cancer Cell 2007; 12: 342–354.
An H, Lu X, Liu D, Yarbrough WG . LZAP inhibits p38 MAPK (p38) phosphorylation and activity by facilitating p38 association with the wild-type p53 induced phosphatase 1 (WIP1). PLoS ONE 2011; 6: e16427.
Abdelmohsen K, Pullmann R Jr, Lal A, Kim HH, Galban S, Yang X et al. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell 2007; 25: 543–557.
Drake JM, Gabriel CL, Henry MD . Assessing tumor growth and distribution in a model of prostate cancer metastasis using bioluminescence imaging. Clin Exp Metastasis 2005; 22: 674–684.
Acknowledgements
We thank W Sun, LA Donehower, N Perkins, Reuven Agami and IM Verma for providing constructs and cell lines. We are extremely grateful to A Richmond, R Matusik, J McLean, A Weaver, D Webb and to Baker Lab members for insightful advice. This work was supported by Grant NIH R01 DE013173 (to WGY) and by funds provided through an endowment from Barry and Amy Baker to the Barry Baker Laboratory for Head & Neck Oncology, from the Vanderbilt Ingram Cancer Center, from the Department of Otolaryngology at Vanderbilt University and from the Vanderbilt Bill Wilkerson Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Lu, X., An, H., Jin, R. et al. PPM1A is a RelA phosphatase with tumor suppressor-like activity. Oncogene 33, 2918–2927 (2014). https://doi.org/10.1038/onc.2013.246
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.246
Keywords
This article is cited by
-
Correlation of PPM1A Downregulation with CYP3A4 Repression in the Tumor Liver Tissue of Hepatocellular Carcinoma Patients
European Journal of Drug Metabolism and Pharmacokinetics (2020)
-
Combined analysis and validation for DNA methylation and gene expression profiles associated with prostate cancer
Cancer Cell International (2019)
-
FHL3 links cell growth and self-renewal by modulating SOX4 in glioma
Cell Death & Differentiation (2019)
-
Hepatitis C virus NS3 protein enhances hepatocellular carcinoma cell invasion by promoting PPM1A ubiquitination and degradation
Journal of Experimental & Clinical Cancer Research (2017)
-
Identification of TWIST-interacting genes in prostate cancer
Science China Life Sciences (2017)