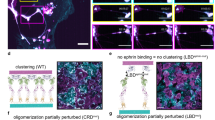
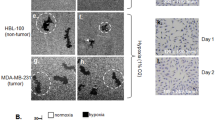
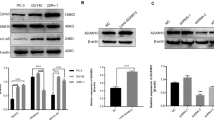
Abstract
Eph receptor tyrosine kinases and their ephrin ligands have been implicated in neuronal development and neovascularization. Overexpression of ephrin-A1 has been implicated in tumor progression and poor prognosis. However, the mechanisms are not clear. Here, we report a role of the Eph/ephrin system in a cell adhesion mechanism. Clustered erythropoietin-producing hepatocellular receptor A1 (EphA1)/ephrin-A1 complexes on the plasma membrane did not undergo endocytosis, and the cell remained adherent to one another. The cell–cell contacts were maintained in an Eph tyrosine kinase activity-independent manner even in the absence of E-cadherin. EphA1 and ephrin-A1 co-localized in pulmonary endothelial cells, and regulated vascular permeability and metastasis in the lungs. We identified ADAM12 (A disintegrin and metalloproteinase 12) as an EphA1-binding partner by yeast two-hybrid screening and found that ADAM12 enhanced ephrin-A1 cleavage in response to transforming growth factor-β1 in primary tumors. Released soluble ephrin-A1 in the serum deteriorated the EphA1/ephrin-A1-mediated cell adhesion in the lungs in an endocrine manner, causing lung hyperpermeability that facilitated tumor cell entry into the lungs. Depletion of soluble ephrin-A1 by its neutralizing antibody significantly inhibited lung metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Pasquale EB . Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer 2010; 10: 165–180.
O'Leary DD, Wilkinson DG . Eph receptors and ephrins in neural development. Curr Opin Neurobiol 1999; 9: 65–73.
Brantley-Sieders DM, Caughron J, Hicks D, Pozzi A, Ruiz JC, Chen J . EphA2 receptor tyrosine kinase regulates endothelial cell migration and vascular assembly through phosphoinositide 3-kinase-mediated Rac1 GTPase activation. J Cell Sci 2004; 117: 2037–2049.
Dodelet VC, Pasquale EB . Eph receptors and ephrin ligands: embryogenesis to tumorigenesis. Oncogene 2000; 19: 5614–5619.
Wykosky J, Debinski W . The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting. Mol Cancer Res 2008; 6: 1795–1806.
Holzman LB, Marks RM, Dixit VM . A novel immediate-early response gene of endothelium is induced by cytokines and encodes a secreted protein. Mol Cell Biol 1990; 10: 5830–5838.
Vihanto MM, Plock J, Erni D, Frey BM, Frey FJ, Huynh-Do U . Hypoxia up-regulates expression of Eph receptors and ephrins in mouse skin. FASEB J 2005; 19: 1689–1691.
Hafner C, Schmitz G, Meyer S, Bataille F, Hau P, Langmann T et al. Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin Chem 2004; 50: 490–499.
Bartley TD, Hunt RW, Welcher AA, Boyle WJ, Parker VP, Lindberg RA et al. B61 is a ligand for the ECK receptor protein-tyrosine kinase. Nature 1994; 368: 558–560.
Masuda J, Usui R, Maru Y . Fibronectin type I repeat is a nonactivating ligand for EphA1 and inhibits ATF3-dependent angiogenesis. J Biol Chem 2008; 283: 13148–13155.
Yamazaki T, Masuda J, Omori T, Usui R, Akiyama H, Maru Y . EphA1 interacts with integrin-linked kinase and regulates cell morphology and motility. J Cell Sci 2009; 122: 243–255.
Larson J, Schomberg S, Schroeder W, Carpenter TC . Endothelial EphA receptor stimulation increases lung vascular permeability. Am J Physiol Lung Cell Mol Physiol 2008; 295: L431–L439.
Okazaki T, Ni A, Baluk P, Ayeni OA, Kearley J, Coyle AJ et al. Capillary defects and exaggerated inflammatory response in the airways of EphA2-deficient mice. Am J Pathol 2009; 174: 2388–2399.
Mochizuki S, Okada Y . ADAMs in cancer cell proliferation and progression. Cancer Sci 2007; 98: 621–628.
Shiomi T, Okada Y . MT1-MMP and MMP-7 in invasion and metastasis of human cancers. Cancer Metastasis Rev 2003; 22: 145–152.
Gilpin BJ, Loechel F, Mattei MG, Engvall E, Albrechtsen R, Wewer UM . A novel, secreted form of human ADAM 12 (meltrin alpha) provokes myogenesis in vivo. J Biol Chem 1998; 273: 157–166.
Asakura M, Kitakaze M, Takashima S, Liao Y, Ishikura F, Yoshinaka T et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med 2002; 8: 35–40.
Roy R, Wewer UM, Zurakowski D, Pories SE, Moses MA . ADAM 12 cleaves extracellular matrix proteins and correlates with cancer status and stage. J Biol Chem 2004; 279: 51323–51330.
Janes PW, Saha N, Barton WA, Kolev MV, Wimmer-Kleikamp SH, Nievergall E et al. Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell 2005; 123: 291–304.
Hattori M, Osterfield M, Flanagan JG . Regulated cleavage of a contact-mediated axon repellent. Science 2000; 289: 1360–1365.
Miao H, Li DQ, Mukherjee A, Guo H, Petty A, Cutter J et al. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell 2009; 16: 9–20.
Aasheim HC, Delabie J, Finne EF . Ephrin-A1 binding to CD4+ T lymphocytes stimulates migration and induces tyrosine phosphorylation of PYK2. Blood 2005; 105: 2869–2876.
Brantley-Sieders DM, Fang WB, Hwang Y, Hicks D, Chen J . Ephrin-A1 facilitates mammary tumor metastasis through an angiogenesis-dependent mechanism mediated by EphA receptor and vascular endothelial growth factor in mice. Cancer Res 2006; 66: 10315–10324.
Davy A, Robbins SM . Ephrin-A5 modulates cell adhesion and morphology in an integrin-dependent manner. EMBO J 2000; 19: 5396–5405.
Davy A, Gale NW, Murray EW, Klinghoffer RA, Soriano P, Feuerstein C et al. Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes Dev 1999; 13: 3125–3135.
Egea J, Klein R . Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol 2007; 17: 230–238.
Hiratsuka S, Goel S, Kamoun WS, Maru Y, Fukumura D, Duda DG et al. Endothelial focal adhesion kinase mediates cancer cell homing to discrete regions of the lungs via E-selectin up-regulation. Proc Natl Acad Sci USA 2010; 108: 3725–3730.
Sundberg C, Thodeti CK, Kveiborg M, Larsson C, Parker P, Albrechtsen R et al. Regulation of ADAM12 cell-surface expression by protein kinase C epsilon. J Biol Chem 2004; 279: 51601–51611.
Beauchamp A, Lively MO, Mintz A, Gibo D, Wykosky J, Debinski W . EphrinA1 is released in three forms from cancer cells by matrix metalloproteases. Mol Cell Biol 2012; 32: 3253–3264.
Blobel CP . Remarkable roles of proteolysis on and beyond the cell surface. Curr Opin Cell Biol 2000; 12: 606–612.
Blobel CP . ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol 2005; 6: 32–43.
Atfi A, Dumont E, Colland F, Bonnier D, L‘Helgoualc’h A, Prunier C et al. The disintegrin and metalloproteinase ADAM12 contributes to TGF-beta signaling through interaction with the type II receptor. J Cell Biol 2007; 178: 201–208.
Le Pabic H, Bonnier D, Wewer UM, Coutand A, Musso O, Baffet G et al. ADAM12 in human liver cancers: TGF-beta-regulated expression in stellate cells is associated with matrix remodeling. Hepatology 2003; 37: 1056–1066.
Kodama T, Ikeda E, Okada A, Ohtsuka T, Shimoda M, Shiomi T et al. ADAM12 is selectively overexpressed in human glioblastomas and is associated with glioblastoma cell proliferation and shedding of heparin-binding epidermal growth factor. Am J Pathol 2004; 165: 1743–1753.
Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron 1996; 17: 9–19.
Brantley-Sieders DM, Fang WB, Hicks DJ, Zhuang G, Shyr Y, Chen J . Impaired tumor microenvironment in EphA2-deficient mice inhibits tumor angiogenesis and metastatic progression. FASEB J 2005; 19: 1884–1886.
Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell 2011; 20: 701–714.
Frohlich C, Nehammer C, Albrechtsen R, Kronqvist P, Kveiborg M, Sehara-Fujisawa A et al. ADAM12 produced by tumor cells rather than stromal cells accelerates breast tumor progression. Mol Cancer Res 2011; 9: 1449–1461.
Wykosky J, Palma E, Gibo DM, Ringler S, Turner CP, Debinski W . Soluble monomeric EphrinA1 is released from tumor cells and is a functional ligand for the EphA2 receptor. Oncogene 2008; 27: 7260–7273.
Alford S, Watson-Hurthig A, Scott N, Carette A, Lorimer H, Bazowski J et al. Soluble ephrin a1 is necessary for the growth of HeLa and SK-BR3 cells. Cancer Cell Int 2010; 10: 41.
Hiratsuka S, Watanabe A, Aburatani H, Maru Y . Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol 2006; 8: 1369–1375.
Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol 2008; 10: 1349–1355.
Yoon S, Woo SU, Kang JH, Kim K, Shin HJ, Gwak HS et al. NF-kappaB and STAT3 cooperatively induce IL6 in starved cancer cells. Oncogene 2012; 19: 3467–3481.
Carpenter TC, Schroeder W, Stenmark KR, Schmidt EP . Eph-A2 promotes permeability and inflammatory responses to bleomycin-induced lung injury. Am J Respir Cell Mol Biol 2012; 46: 40–47.
Orsulic S, Kemler R . Expression of Eph receptors and ephrins is differentially regulated by E-cadherin. J Cell Sci 2000; 113 (Pt 10): 1793–1802.
Tanaka M, Kamata R, Sakai R . EphA2 phosphorylates the cytoplasmic tail of Claudin-4 and mediates paracellular permeability. J Biol Chem 2005; 280: 42375–42382.
Naruse-Nakajima C, Asano M, Iwakura Y . Involvement of EphA2 in the formation of the tail notochord via interaction with ephrinA1. Mech Dev 2001; 102: 95–105.
Duffy SL, Coulthard MG, Spanevello MD, Herath NI, Yeadon TM, McCarron JK et al. Generation and characterization of EphA1 receptor tyrosine kinase reporter knockout mice. Genesis 2008; 46: 553–561.
Ieguchi K, Ueda S, Kataoka T, Satoh T . Role of the guanine nucleotide exchange factor Ost in negative regulation of receptor endocytosis by the small GTPase Rac1. J Biol Chem 2007; 282: 23296–23305.
Ieguchi K, Fujita M, Ma Z, Davari P, Taniguchi Y, Sekiguchi K et al. Direct binding of the EGF-like domain of neuregulin-1 to integrins ({alpha}v{beta}3 and {alpha}6{beta}4) is involved in neuregulin-1/ErbB signaling. J Biol Chem 2010; 285: 31388–31398.
Acknowledgements
We thank Dr Yoichiro Iwakura at the University of Tokyo for providing EphA2-KO mice, Dr Ulla Wewer at the University of Copenhagen for providing human ADAM12 cDNA clones and Ms. Helen Jeays at the Queensland Institute of Medical Research for proofreading. This work was supported by grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, grant nos. 21117008 (to YM), 23590347 (to TT), 25122716 and 25870760 (to KI), and the Global COE program, Multidisciplinary Education and Research Center for Regenerative Medicine from Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Ieguchi, K., Tomita, T., Omori, T. et al. ADAM12-cleaved ephrin-A1 contributes to lung metastasis. Oncogene 33, 2179–2190 (2014). https://doi.org/10.1038/onc.2013.180
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.180
Keywords
This article is cited by
-
Beyond the barrier: the immune-inspired pathways of tumor extravasation
Cell Communication and Signaling (2024)
-
Eph receptors and ephrins in cancer progression
Nature Reviews Cancer (2024)
-
An integrated analysis of single-cell and bulk transcriptomics reveals EFNA1 as a novel prognostic biomarker for cervical cancer
Human Cell (2022)
-
Human ribonuclease 1 serves as a secretory ligand of ephrin A4 receptor and induces breast tumor initiation
Nature Communications (2021)
-
C1GALT1 is associated with poor survival and promotes soluble Ephrin A1-mediated cell migration through activation of EPHA2 in gastric cancer
Oncogene (2020)