Abstract
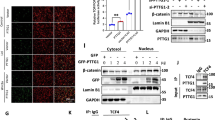
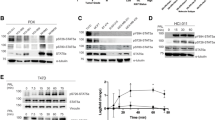
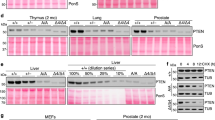
Pituitary tumor-transforming gene (PTTG), the index mammalian securin, is abundantly expressed in several tumors and regulates tumor growth and progression. Molecular mechanisms elucidating PTTG regulation and actions remain elusive. Here, we provide evidence that PTTG acts as a signal transducer and activator of transcription factor 3 (STAT3) target gene. Total STAT3 and Tyr705 phosphorylated STAT3 were concordantly expressed with PTTG in human colorectal tumors (n=97 and n=95, respectively, P<0.001). STAT3 specifically bound the human PTTG promoter and induced PTTG transcriptional activity (twofold) as assessed by chromatin immunoprecipitation and luciferase reporter assays. STAT3 transfection increased PTTG mRNA and protein abundance twofold in HCT116 human colon cancer cells, and induction was further enhanced (threefold) by constitutively active STAT3 (STAT3-C), whereas strongly abrogated by dominant-negative STAT3 (STAT3-DN). Attenuating PTTG expression by siRNA in STAT3 HCT116 stable transfectants suppressed cell growth and colony formation in vitro, and PTTG cell knockout also constrained activated STAT3-induced explanted murine tumor growth in vivo. STAT3 increased HCT116 cell migration and invasion up to fivefold, whereas cell mobility was abolished by STAT3-DN (>85%). Impairing PTTG expression by siRNA also strongly suppressed STAT3-faciliated cell migration and invasion by up to 90%. Knocking out PTTG in STAT3-C HCT116 stable transfectants strongly decreased tumor metastases in nude mice, indicating the requirement of PTTG for STAT3-promoted metastasis. These results elucidate a mechanism for tumor cell PTTG regulation, whereby STAT3 induces PTTG expression to facilitate tumor growth and metastasis, and further support the rationale for targeting PTTG to abrogate colorectal cancer growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Buettner R, Mora LB, Jove R . Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res 2002; 8: 945–954.
Vlotides G, Eigler T, Melmed S . Pituitary tumor-transforming gene: physiology and implications for tumorigenesis. Endocr Rev 2007; 28: 165–186.
Pei L, Melmed S . Isolation and characterization of a pituitary tumor-transforming gene (PTTG). Mol Endocrinol 1997; 11: 433–441.
Zou H, McGarry TJ, Bernal T, Kirschner MW . Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science 1999; 285: 418–422.
Jallepalli PV, Waizenegger IC, Bunz F, Langer S, Speicher MR, Peters JM et al. Securin is required for chromosomal stability in human cells. Cell 2001; 105: 445–457.
Romero F, Multon MC, Ramos-Morales F, Dominguez A, Bernal JA, Pintor-Toro JA et al. Human securin, hPTTG, is associated with Ku heterodimer, the regulatory subunit of the DNA-dependent protein kinase. Nucleic Acids Res 2001; 29: 1300–1307.
Romero F, Gil-Bernabe AM, Saez C, Japon MA, Pintor-Toro JA, Tortolero M . Securin is a target of the UV response pathway in mammalian cells. Mol Cell Biol 2004; 24: 2720–2733.
Zhang X, Horwitz GA, Prezant TR, Valentini A, Nakashima M, Bronstein MD et al. Structure, expression, and function of human pituitary tumor-transforming gene (PTTG). Mol Endocrinol 1999; 13: 156–166.
Heaney AP, Singson R, McCabe CJ, Nelson V, Nakashima M, Melmed S . Expression of pituitary-tumour transforming gene in colorectal tumours. Lancet 2000; 355: 716–719.
Zhang X, Horwitz GA, Heaney AP, Nakashima M, Prezant TR, Bronstein MD et al. Pituitary tumor transforming gene (PTTG) expression in pituitary adenomas. J Clin Endocrinol Metab 1999; 84: 761–767.
Filippella M, Galland F, Kujas M, Young J, Faggiano A, Lombardi G et al. Pituitary tumour transforming gene (PTTG) expression correlates with the proliferative activity and recurrence status of pituitary adenomas: a clinical and immunohistochemical study. Clin Endocrinol (Oxf) 2006; 65: 536–543.
Boelaert K, McCabe CJ, Tannahill LA, Gittoes NJ, Holder RL, Watkinson JC et al. Pituitary tumor transforming gene and fibroblast growth factor-2 expression: potential prognostic indicators in differentiated thyroid cancer. J Clin Endocrinol Metab 2003; 88: 2341–2347.
Ogbagabriel S, Fernando M, Waldman FM, Bose S, Heaney AP . Securin is overexpressed in breast cancer. Mod Pathol 2005; 18: 985–990.
Zhou C, Liu S, Zhou X, Xue L, Quan L, Lu N et al. Overexpression of human pituitary tumor transforming gene (hPTTG), is regulated by beta-catenin/TCF pathway in human esophageal squamous cell carcinoma. Int J Cancer 2005; 113: 891–898.
Salehi F, Kovacs K, Scheithauer BW, Lloyd RV, Cusimano M . Pituitary tumor-transforming gene in endocrine and other neoplasms: a review and update. Endocr Relat Cancer 2008; 15: 721–743.
Panguluri SK, Yeakel C, Kakar SS . PTTG: an important target gene for ovarian cancer therapy. J Ovarian Res 2008; 1: 6.
Ramaswamy S, Ross KN, Lander ES, Golub TR . A molecular signature of metastasis in primary solid tumors. Nat Genet 2003; 33: 49–54.
Yan S, Zhou C, Lou X, Xiao Z, Zhu H, Wang Q et al. PTTG overexpression promotes lymph node metastasis in human esophageal squamous cell carcinoma. Cancer Res 2009; 69: 3283–3290.
Yu R, Lu W, Chen J, McCabe CJ, Melmed S . Overexpressed pituitary tumor-transforming gene causes aneuploidy in live human cells. Endocrinology 2003; 144: 4991–4998.
Pei L . Identification of c-myc as a down-stream target for pituitary tumor-transforming gene. J Biol Chem 2001; 276: 8484–8491.
Tong Y, Tan Y, Zhou C, Melmed S . Pituitary tumor transforming gene interacts with Sp1 to modulate G1/S cell phase transition. Oncogene 2007; 26: 5596–5605.
Kim CS, Ying H, Willingham MC, Cheng SY . The pituitary tumor-transforming gene promotes angiogenesis in a mouse model of follicular thyroid cancer. Carcinogenesis 2007; 28: 932–939.
Hamid T, Malik MT, Kakar SS . Ectopic expression of PTTG1/securin promotes tumorigenesis in human embryonic kidney cells. Mol Cancer 2005; 4: 3.
Zhou C, Wawrowsky K, Bannykh S, Gutman S, Melmed S . E2F1 induces pituitary tumor transforming gene (PTTG1) expression in human pituitary tumors. Mol Endocrinol 2009; 23: 2000–2012.
Sasse J, Hemmann U, Schwartz C, Schniertshauer U, Heesel B, Landgraf C et al. Mutational analysis of acute-phase response factor/Stat3 activation and dimerization. Mol Cell Biol 1997; 17: 4677–4686.
Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C et al. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann NY Acad Sci 2009; 1171: 59–76.
Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C et al. Stat3 as an oncogene. Cell 1999; 98: 295–303.
Morikawa T, Baba Y, Yamauchi M, Kuchiba A, Nosho K, Shima K et al. STAT3 expression, molecular features, inflammation patterns, and prognosis in a database of 724 colorectal cancers. Clin Cancer Res 2011; 17: 1452–1462.
Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA et al. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene 2001; 20: 2499–2513.
Siddiquee K, Zhang S, Guida WC, Blaskovich MA, Greedy B, Lawrence HR et al. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci USA 2007; 104: 7391–7396.
Lin L, Amin R, Gallicano GI, Glasgow E, Jogunoori W, Jessup JM et al. The STAT3 inhibitor NSC 74859 is effective in hepatocellular cancers with disrupted TGF-beta signaling. Oncogene 2009; 28: 961–972.
Bowman T, Broome MA, Sinibaldi D, Wharton W, Pledger WJ, Sedivy JM et al. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc Natl Acad Sci USA 2001; 98: 7319–7324.
Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y, Azuma K et al. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res 2002; 62: 3351–3355.
Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 2002; 21: 2000–2008.
Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F, Sawaya R et al. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene 2004; 23: 3550–3560.
Zhou C, Tong Y, Wawrowsky K, Bannykh S, Donangelo I, Melmed S . Oct-1 induces pituitary tumor transforming gene expression in endocrine tumors. Endocr Relat Cancer 2008; 15: 817–831.
Wicki A, Herrmann R, Christofori G . Kras in metastatic colorectal cancer. Swiss Med Wkly 2010; 140: w13112.
Horsch M, Recktenwald CV, Schadler S, Hrabe de Angelis M, Seliger B, Beckers J . Overexpressed vs mutated Kras in murine fibroblasts: a molecular phenotyping study. Br J Cancer 2009; 100: 656–662.
Sano S, Itami S, Takeda K, Tarutani M, Yamaguchi Y, Miura H et al. Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO J 1999; 18: 4657–4668.
Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G et al. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell 2006; 126: 489–502.
Silver DL, Naora H, Liu J, Cheng W, Montell DJ . Activated signal transducer and activator of transcription (STAT) 3: localization in focal adhesions and function in ovarian cancer cell motility. Cancer Res 2004; 64: 3550–3558.
Ng DC, Lin BH, Lim CP, Huang G, Zhang T, Poli V et al. Stat3 regulates microtubules by antagonizing the depolymerization activity of stathmin. J Cell Biol 2006; 172: 245–257.
Westermarck J, Kahari VM . Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J 1999; 13: 781–792.
Song Y, Qian L, Song S, Chen L, Zhang Y, Yuan G et al. Fra-1 and Stat3 synergistically regulate activation of human MMP-9 gene. Mol Immunol 2008; 45: 137–143.
Tsareva SA, Moriggl R, Corvinus FM, Wiederanders B, Schutz A, Kovacic B et al. Signal transducer and activator of transcription 3 activation promotes invasive growth of colon carcinomas through matrix metalloproteinase induction. Neoplasia 2007; 9: 279–291.
Malik MT, Kakar SS . Regulation of angiogenesis and invasion by human Pituitary tumor transforming gene (PTTG) through increased expression and secretion of matrix metalloproteinase-2 (MMP-2). Mol Cancer 2006; 5: 61.
Acknowledgements
We are grateful to Dr Bert Vogelstein at Johns Hopkins University (Baltimore, MD, USA) for kindly providing HCT116 PTTG+/+ and HCT116 PTTG−/− cells; and thank Patricia Lin at the Cedars-Sinai Flow Cytometry Core. This work was supported by National Institutes of Health Grant CA75979 (to SM) and the Doris Factor Molecular Endocrinology Laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhou, C., Tong, Y., Wawrowsky, K. et al. PTTG acts as a STAT3 target gene for colorectal cancer cell growth and motility. Oncogene 33, 851–861 (2014). https://doi.org/10.1038/onc.2013.16
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.16
Keywords
This article is cited by
-
The JAK2/STAT3/CCND2 Axis promotes colorectal Cancer stem cell persistence and radioresistance
Journal of Experimental & Clinical Cancer Research (2019)
-
Subverted regulation of Nox1 NADPH oxidase-dependent oxidant generation by protein disulfide isomerase A1 in colon carcinoma cells with overactivated KRas
Cell Death & Disease (2019)
-
Targeting colon cancer with the novel STAT3 inhibitor bruceantinol
Oncogene (2019)
-
FoxM1 transactivates PTTG1 and promotes colorectal cancer cell migration and invasion
BMC Medical Genomics (2015)
-
Glutamate dehydrogenase is a novel prognostic marker and predicts metastases in colorectal cancer patients
Journal of Translational Medicine (2015)