Abstract
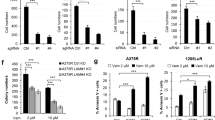
Elevated activity of the mitogen-activated protein kinase (MAPK) signaling cascade is found in the majority of human melanomas and is known to regulate proliferation, survival and invasion. Current targeted therapies focus on decreasing the activity of this pathway; however, we do not fully understand how these therapies impact tumor biology, especially given that melanoma is a heterogeneous disease. Using a three-dimensional (3D), collagen-embedded spheroid melanoma model, we observed that MEK and BRAF inhibitors can increase the invasive potential of ∼20% of human melanoma cell lines. The invasive cell lines displayed increased receptor tyrosine kinase (RTK) activity and activation of the Src/FAK/signal transducers and activators of transcription-3 (STAT3) signaling axis, also associated with increased cell-to-cell adhesion and cadherin engagement following MEK inhibition. Targeting various RTKs, Src, FAK and STAT3 with small molecule inhibitors in combination with a MEK inhibitor prevented the invasive phenotype, but only STAT3 inhibition caused cell death in the 3D context. We further show that STAT3 signaling is induced in BRAF-inhibitor-resistant cells. Our findings suggest that MEK and BRAF inhibitors can induce STAT3 signaling, causing potential adverse effects such as increased invasion. We also provide the rationale for the combined targeting of the MAPK pathway along with inhibitors of RTKs, SRC or STAT3 to counteract STAT3-mediated resistance phenotypes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Haass NK, Smalley KS, Herlyn M . The role of altered cell-cell communication in melanoma progression. J Mol Histol 2004; 35: 309–318.
Fecher LA, Cummings SD, Keefe MJ, Alani RM . Toward a molecular classification of melanoma. J Clin Oncol 2007; 25: 1606–1620.
Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 2010; 467: 596–599.
Lee JT, Li L, Brafford PA, van den Eijnden M, Halloran MB, Sproesser K et al. PLX4032, a potent inhibitor of the B-Raf V600E oncogene, selectively inhibits V600E-positive melanomas. Pigment Cell Melanoma Res 2010; 23: 820–827.
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364: 2507–2516.
Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 2012; 366: 707–714.
Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 2010; 363: 809–819.
Halaban R, Zhang W, Bacchiocchi A, Cheng E, Parisi F, Ariyan S et al. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res 2010; 23: 190–200.
Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell 2010; 18: 683–695.
Johannessen CMBJ, Kim SY, Thomas SR, Wardwell L, Johnson LA, Emery CM et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 2010; 468: 968–972.
Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N . RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 2010; 464: 427–430.
Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 2010; 140: 209–221.
Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature 2010; 464: 431–435.
Nazarian RSH, Wang Q, Kong X, Koya RC, Lee H, Chen Z et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 2010; 468: 973–977.
Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 2012; 487: 500–504.
Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature 2012; 487: 505–509.
Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol 2011; 29: 3085–3096.
Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 2010; 468: 968–972.
Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature 2006; 439: 358–362.
Dry JR, Pavey S, Pratilas CA, Harbron C, Runswick S, Hodgson D et al. Transcriptional pathway signatures predict MEK addiction and response to selumetinib (AZD6244). Cancer Res 2010; 70: 2264–2273.
Nissan MH, Solit DB . The "SWOT" of BRAF inhibition in melanoma: RAF inhibitors, MEK inhibitors or both? Curr Oncol Rep 2011; 13: 479–487.
Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012; 367: 107–114.
Gowrishankar K, Snoyman S, Pupo GM, Becker TM, Kefford RF, Rizos H . Acquired resistance to BRAF inhibition can confer cross-resistance to combined BRAF/MEK inhibition. J Invest Dermatol 2012; 132: 1850–1859.
Smalley KS, Haass NK, Brafford PA, Lioni M, Flaherty KT, Herlyn M . Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Mol Cancer Ther 2006; 5: 1136–1144.
Shi H, Kong X, Ribas A, Lo RS . Combinatorial treatments that overcome PDGFR{beta}-driven resistance of melanoma cells to V600EB-RAF inhibition. Cancer Res 2011; 71: 5067–5074.
Gopal YN, Deng W, Woodman SE, Komurov K, Ram P, Smith PD et al. Basal and treatment-induced activation of AKT mediates resistance to cell death by AZD6244 (ARRY-142886) in Braf-mutant human cutaneous melanoma cells. Cancer Res 2010; 70: 8736–8747.
Greger JG, Eastman SD, Zhang V, Bleam MR, Hughes AM, Smitheman KN et al. Combinations of BRAF, MEK, and PI3K/mTOR inhibitors overcome acquired resistance to the BRAF inhibitor GSK2118436 dabrafenib, mediated by NRAS or MEK mutations. Mol Cancer Ther 2012; 11: 909–920.
Haass NK, Sproesser K, Nguyen TK, Contractor R, Medina CA, Nathanson KL et al. The mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitor AZD6244 (ARRY-142886) induces growth arrest in melanoma cells and tumor regression when combined with docetaxel. Clin Cancer Res 2008; 14: 230–239.
Zong CS, Chan J, Levy DE, Horvath C, Sadowski HB, Wang LH . Mechanism of STAT3 activation by insulin-like growth factor I receptor. J Biol Chem 2000; 275: 15099–15105.
Wang YZ, Wharton W, Garcia R, Kraker A, Jove R, Pledger WJ . Activation of Stat3 preassembled with platelet-derived growth factor beta receptors requires Src kinase activity. Oncogene 2000; 19: 2075–2085.
Dudka AA, Sweet SM, Heath JK . Signal transducers and activators of transcription-3 binding to the fibroblast growth factor receptor is activated by receptor amplification. Cancer Res 2010; 70: 3391–3401.
Niu G, Bowman T, Huang M, Shivers S, Reintgen D, Daud A et al. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene 2002; 21: 7001–7010.
Vultur A, Cao J, Arulanandam R, Turkson J, Jove R, Greer P et al. Cell-to-cell adhesion modulates Stat3 activity in normal and breast carcinoma cells. Oncogene 2004; 23: 2600–2616.
Littlefield SL, Baird MC, Anagnostopoulou A, Raptis L . Synthesis, characterization and Stat3 inhibitory properties of the prototypical platinum(IV) anticancer drug, [PtCl3(NO2)(NH3)2] (CPA-7). Inorg Chem 2008; 47: 2798–2804.
Zipser MC, Eichhoff OM, Widmer DS, Schlegel NC, Schoenewolf NL, Stuart D et al. A proliferative melanoma cell phenotype is responsive to RAF/MEK inhibition independent of BRAF mutation status. Pigment Cell Melanoma Res 2011; 24: 326–333.
Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS . Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 2009; 15: 232–239.
Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 2009; 15: 220–231.
Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell 2010; 18: 683–695.
Debidda M, Wang L, Zang H, Poli V, Zheng Y . A role of STAT3 in Rho GTPase-regulated cell migration and proliferation. J Biol Chem 2005; 280: 17275–17285.
Arulanandam R, Vultur A, Cao J, Carefoot E, Elliott BE, Truesdell PF et al. Cadherin-cadherin engagement promotes cell survival via Rac1/Cdc42 and signal transducer and activator of transcription-3. Mol Cancer Res 2009; 7: 1310–1327.
Christofori G . Changing neighbours, changing behaviour: cell adhesion molecule-mediated signalling during tumour progression. EMBO J 2003; 22: 2318–2323.
Weng YI, Aroor AR, Shukla SD . Ethanol inhibition of angiotensin II-stimulated Tyr705 and Ser727 STAT3 phosphorylation in cultured rat hepatocytes: relevance to activation of p42/44 mitogen-activated protein kinase. Alcohol 2008; 42: 397–406.
Sakaguchi M, Oka M, Iwasaki T, Fukami Y, Nishigori C . Role and regulation of STAT3 phosphorylation at Ser727 in melanocytes and melanoma cells. J Invest Dermatol 2012; 132: 1877–1885.
Buettner R, Mesa T, Vultur A, Lee F, Jove R . Inhibition of Src family kinases with dasatinib blocks migration and invasion of human melanoma cells. Mol Cancer Res 2008; 6: 1766–1774.
Ferguson J, Arozarena I, Ehrhardt M, Wellbrock C . Combination of MEK and SRC inhibition suppresses melanoma cell growth and invasion. Oncogene 2012; 32: 86–96.
Senft C, Priester M, Polacin M, Schroder K, Seifert V, Kogel D et al. Inhibition of the JAK-2/STAT3 signaling pathway impedes the migratory and invasive potential of human glioblastoma cells. J Neurooncol 2011; 101: 393–403.
Satyamoorthy K, Li G, Gerrero MR, Brose MS, Volpe P, Weber BL et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res 2003; 63: 756–759.
Iliopoulos D, Ernst C, Steplewski Z, Jambrosic JA, Rodeck U, Herlyn M et al. Inhibition of metastases of a human melanoma xenograft by monoclonal antibody to the GD2/GD3 gangliosides. J Natl Cancer Inst 1989; 81: 440–444.
Thomas RK, Baker AC, Debiasi RM, Winckler W, Laframboise T, Lin WM et al. High-throughput oncogene mutation profiling in human cancer. Nat Genet 2007; 39: 347–351.
Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell 2009; 16: 21–32.
Ragoussis J, Elvidge GP, Kaur K, Colella S . Matrix-assisted laser desorption/ionisation, time-of-flight mass spectrometry in genomics research. PLoS Genet 2006; 2: e100.
MacConaill LE, Campbell CD, Kehoe SM, Bass AJ, Hatton C, Niu L et al. Profiling critical cancer gene mutations in clinical tumor samples. PLoS One 2009; 4: e7887.
Smalley KS, Herlyn M . Targeting intracellular signaling pathways as a novel strategy in melanoma therapeutics. Ann NY Acad Sci 2005; 1059: 16–25.
Smalley KS, Contractor R, Nguyen TK, Xiao M, Edwards R, Muthusamy V et al. Identification of a novel subgroup of melanomas with KIT/cyclin-dependent kinase-4 overexpression. Cancer Res 2008; 68: 5743–5752.
Vultur A, Buettner R, Kowolik C, Liang W, Smith D, Boschelli F et al. SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells. Mol Cancer Ther 2008; 7: 1185–1194.
Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell 2010; 141: 583–594.
Fukunaga-Kalabis M, Martinez G, Liu ZJ, Kalabis J, Mrass P, Weninger W et al. CCN3 controls 3D spatial localization of melanocytes in the human skin through DDR1. J Cell Biol 2006; 175: 563–569.
Acknowledgements
We thank P Sompalli and F Raman for technical assistance. We thank GlaxoSmithKline for SB590885, and Dr L Raptis (Queen’s University, Canada) for CPA-7. We also thank R Delgiacco of the Wistar Histology Facility. AV, JV, KLN and MH are members of the ITMAT–University of Pennsylvania. This work was supported by NIH grants PO1 CA114046, P01 CA025874, P30 CA010815, R01 CA047159 and CA076674, and by the Dr Miriam and Sheldon G. Adelson Medical Research Foundation (AMRF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Vultur, A., Villanueva, J., Krepler, C. et al. MEK inhibition affects STAT3 signaling and invasion in human melanoma cell lines. Oncogene 33, 1850–1861 (2014). https://doi.org/10.1038/onc.2013.131
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.131
Keywords
This article is cited by
-
STAT3 transcription factor as target for anti-cancer therapy
Pharmacological Reports (2020)
-
STAT3 enhances the constitutive activity of AGC kinases in melanoma by transactivating PDK1
Cell & Bioscience (2019)
-
Phosphoprotein patterns predict trametinib responsiveness and optimal trametinib sensitisation strategies in melanoma
Cell Death & Differentiation (2019)
-
Targeting colon cancer with the novel STAT3 inhibitor bruceantinol
Oncogene (2019)
-
Abl kinase regulation by BRAF/ERK and cooperation with Akt in melanoma
Oncogene (2017)