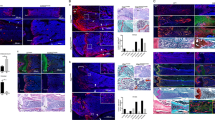
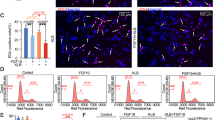
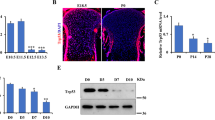
Abstract
Cell proliferation and differentiation are closely coupled. However, we previously showed that overexpression of cyclin-dependent kinase (Cdk6) blocks chondrocyte differentiation without affecting cell-cycle progression in vitro. To investigate whether Cdk6 inhibits chondrocyte differentiation in vivo, we generated chondrocyte-specific Cdk6 transgenic mice using Col2a1 promoter. Unexpectedly, differentiation and cell-cycle progression of chondrocytes in the Cdk6 transgenic mice were similar to those in wild-type mice. Then, we generated chondrocyte-specific Ccnd1 transgenic mice and Cdk6/Ccnd1 double transgenic mice to investigate the possibility that Cdk6 inhibits chondrocyte differentiation through E2f activation. Bromodeoxyuridine (BrdU)-positive chondrocytes and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive chondrocytes were increased in number, and chondrocyte maturation was inhibited only in Cdk6/Ccnd1 transgenic mice (K6H/D1H mice), which showed dwarfism. Retinoblastoma protein (pRb) was highly phosphorylated but p107 was upregulated, and the expression of E2f target genes was dysregulated as shown by upregulation of Cdc6 but downregulation of cyclin E, dihydrofolate reductase (dhfr), Cdc25a and B-Myb in chondrocytes of K6H/D1H mice. Similarly, overexpression of Cdk6/Ccnd1 in a chondrogenic cell line ATDC5 highly phosphorylated pRb, upregulated p107, induced apoptosis, upregulated Cdc6 and downregulated cyclin E, dhfr and B-Myb and p107 small interfering RNA reversed the expression of downregulated genes. Further, introduction of kinase-negative Cdk6 and cyclin D1 abolished all effects by Cdk6/cyclin D1 in ATDC5 cells, indicating the requirement of the kinase activity on these effects. p53 deletion partially restored the size of the skeleton and almost completely rescued chondrocyte apoptosis, but failed to enhance chondrocyte proliferation in K6H/D1H mice. These findings indicated that Cdk6/Ccnd1 overexpression inhibited chondrocyte maturation and enhanced G1/S cell-cycle transition by phosphorylating pRb, but the chondrocytes failed to accomplish the cell cycle, and underwent p53-dependent apoptosis probably due to the dysregulation of E2f target genes. Our findings also indicated that p53 deletion in addition to the inactivation of Rb was not sufficient to accelerate chondrocyte proliferation, suggesting the resistance of chondrocytes to sarcomagenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kronenberg HM . Developmental regulation of the growth plate. Nature 2003; 423: 332–336.
Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL . Cyclin D as a therapeutic target in cancer. Nat Rev Cancer 2011; 11: 558–572.
Beier F, Ali Z, Mok D, Taylor AC, Leask T, Albanese C et al. TGFbeta and PTHrP control chondrocyte proliferation by activating cyclin D1 expression. Mol Biol Cell 2001; 12: 3852–3863.
Long F, Zhang XM, Karp S, Yang Y, McMahon AP . Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development 2001; 128: 5099–5108.
Yang Y, Topol L, Lee H, Wu J . Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development 2003; 130: 1003–1015.
Grashoff C, Aszodi A, Sakai T, Hunziker EB, Fassler R . Integrin-linked kinase regulates chondrocyte shape and proliferation. EMBO Rep 2003; 4: 432–438.
Terpstra L, Prud'homme J, Arabian A, Takeda S, Karsenty G, Dedhar S et al. Reduced chondrocyte proliferation and chondrodysplasia in mice lacking the integrin-linked kinase in chondrocytes. J Cell Biol 2003; 162: 139–148.
Wang G, Woods A, Sabari S, Pagnotta L, Stanton LA, Beier F . RhoA/ROCK signaling suppresses hypertrophic chondrocyte differentiation. J Biol Chem 2004; 279: 13205–13214.
Sunters A, McCluskey J, Grigoriadis AE . Control of cell cycle gene expression in bone development and during c-Fos-induced osteosarcoma formation. Dev Genet 1998; 22: 386–397.
Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C . Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev 1995; 9: 2364–2372.
Sicinski P, Donaher JL, Parker SB, Li T, Fazeli A, Gardner H et al. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 1995; 82: 621–630.
Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV . Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature 1994; 369: 669–671.
Mueller A, Odze R, Jenkins TD, Shahsesfaei A, Nakagawa H, Inomoto T et al. A transgenic mouse model with cyclin D1 overexpression results in cell cycle, epidermal growth factor receptor, and p53 abnormalities. Cancer Res 1997; 57: 5542–5549.
Lovec H, Grzeschiczek A, Kowalski MB, Moroy T . Cyclin D1/bcl-1 cooperates with myc genes in the generation of B-cell lymphoma in transgenic mice. EMBO J 1994; 13: 3487–3495.
Garcia-Espana A, Salazar E, Sun TT, Wu XR, Pellicer A . Differential expression of cell cycle regulators in phenotypic variants of transgenically induced bladder tumors: implications for tumor behavior. Cancer Res 2005; 65: 1150–1157.
Resnitzky D, Gossen M, Bujard H, Reed SI . Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol 1994; 14: 1669–1679.
Quelle DE, Ashmun RA, Shurtleff SA, Kato JY, Bar-Sagi D, Roussel MF et al. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev 1993; 7: 1559–1571.
Jiang W, Kahn SM, Zhou P, Zhang YJ, Cacace AM, Infante AS et al. Overexpression of cyclin D1 in rat fibroblasts causes abnormalities in growth control, cell cycle progression and gene expression. Oncogene 1993; 8: 3447–3457.
Cobrinik D, Lee MH, Hannon G, Mulligan G, Bronson RT, Dyson N et al. Shared role of the pRB-related p130 and p107 proteins in limb development. Genes Dev 1996; 10: 1633–1644.
Rossi F, MacLean HE, Yuan W, Francis RO, Semenova E, Lin CS et al. p107 and p130 Coordinately regulate proliferation, Cbfa1 expression, and hypertrophic differentiation during endochondral bone development. Dev Biol 2002; 247: 271–285.
Moro T, Ogasawara T, Chikuda H, Ikeda T, Ogata N, Maruyama Z et al. Inhibition of Cdk6 expression through p38 MAP kinase is involved in differentiation of mouse prechondrocyte ATDC5. J Cell Physiol 2005; 204: 927–933.
Ueta C, Iwamoto M, Kanatani N, Yoshida C, Liu Y, Enomoto-Iwamoto M et al. Skeletal malformations caused by overexpression of Cbfa1 or its dominant negative form in chondrocytes. J Cell Biol 2001; 153: 87–100.
Ojala PM, Yamamoto K, Castanos-Velez E, Biberfeld P, Korsmeyer SJ, Makela TP . The apoptotic v-cyclin-CDK6 complex phosphorylates and inactivates Bcl-2. Nat Cell Biol 2000; 2: 819–825.
Hurford RK Jr, Cobrinik D, Lee MH, Dyson N . pRB and p107/p130 are required for the regulated expression of different sets of E2F responsive genes. Genes Dev 1997; 11: 1447–1463.
Wells J, Boyd KE, Fry CJ, Bartley SM, Farnham PJ . Target gene specificity of E2F and pocket protein family members in living cells. Mol Cell Biol 2000; 20: 5797–5807.
Vigo E, Muller H, Prosperini E, Hateboer G, Cartwright P, Moroni MC et al. CDC25A phosphatase is a target of E2F and is required for efficient E2F-induced S phase. Mol Cell Biol 1999; 19: 6379–6395.
Chen Q, Lin J, Jinno S, Okayama H . Overexpression of Cdk6-cyclin D3 highly sensitizes cells to physical and chemical transformation. Oncogene 2003; 22: 992–1001.
Hartwell L . Defects in a cell cycle checkpoint may be responsible for the genomic instability of cancer cells. Cell 1992; 71: 543–546.
Ohnuma S, Philpott A, Wang K, Holt CE, Harris WA . p27Xic1, a Cdk inhibitor, promotes the determination of glial cells in Xenopus retina. Cell 1999; 99: 499–510.
Erhardt JA, Pittman RN . Ectopic p21(WAF1) expression induces differentiation-specific cell cycle changes in PC12 cells characteristic of nerve growth factor treatment. J Biol Chem 1998; 273: 23517–23523.
Matsumura I, Ishikawa J, Nakajima K, Oritani K, Tomiyama Y, Miyagawa J et al. Thrombopoietin-induced differentiation of a human megakaryoblastic leukemia cell line, CMK, involves transcriptional activation of p21(WAF1/Cip1) by STAT5. Mol Cell Biol 1997; 17: 2933–2943.
Liu M, Lee MH, Cohen M, Bommakanti M, Freedman LP . Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev 1996; 10: 142–153.
Zhang P, Liegeois NJ, Wong C, Finegold M, Hou H, Thompson JC et al. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature 1997; 387: 151–158.
Yan Y, Frisen J, Lee MH, Massague J, Barbacid M . Ablation of the CDK inhibitor p57Kip2 results in increased apoptosis and delayed differentiation during mouse development. Genes Dev 1997; 11: 973–983.
Takahashi K, Nakayama K . Mice lacking a CDK inhibitor, p57Kip2, exhibit skeletal abnormalities and growth retardation. J Biochem 2000; 127: 73–83.
Fiaschi-Taesch NM, Salim F, Kleinberger J, Troxell R, Cozar-Castellano I, Selk K et al. Induction of human beta-cell proliferation and engraftment using a single G1/S regulatory molecule, cdk6. Diabetes 2010; 59: 1926–1936.
Cocker JH, Piatti S, Santocanale C, Nasmyth K, Diffley JF . An essential role for the Cdc6 protein in forming the pre-replicative complexes of budding yeast. Nature 1996; 379: 180–182.
Tanaka T, Knapp D, Nasmyth K . Loading of an Mcm protein onto DNA replication origins is regulated by Cdc6p and CDKs. Cell 1997; 90: 649–660.
Schnell JR, Dyson HJ, Wright PE . Structure, dynamics, and catalytic function of dihydrofolate reductase. Annu Rev Biophys Biomol Struct 2004; 33: 119–140.
Burkhart DL, Wirt SE, Zmoos AF, Kareta MS, Sage J . Tandem E2F binding sites in the promoter of the p107 cell cycle regulator control p107 expression and its cellular functions. PLoS Genet 2010; 6: e1001003.
Lowe SW, Sherr CJ . Tumor suppression by Ink4a-Arf: progress and puzzles. Curr Opin Genet Dev 2003; 13: 77–83.
Harris SL, Levine AJ . The p53 pathway: positive and negative feedback loops. Oncogene 2005; 24: 2899–2908.
Innocente SA, Abrahamson JL, Cogswell JP, Lee JM . p53 regulates a G2 checkpoint through cyclin B1. Proc Natl Acad Sci USA 1999; 96: 2147–2152.
Taylor WR, DePrimo SE, Agarwal A, Agarwal ML, Schonthal AH, Katula KS et al. Mechanisms of G2 arrest in response to overexpression of p53. Mol Biol Cell 1999; 10: 3607–3622.
Li M, Lee H, Yoon DW, Albrecht JC, Fleckenstein B, Neipel F et al. Kaposi’s sarcoma-associated herpesvirus encodes a functional cyclin. J Virol 1997; 71: 1984–1991.
Ojala PM, Tiainen M, Salven P, Veikkola T, Castanos-Velez E, Sarid R et al. Kaposi’s sarcoma-associated herpesvirus-encoded v-cyclin triggers apoptosis in cells with high levels of cyclin-dependent kinase 6. Cancer Res 1999; 59: 4984–4989.
Verschuren EW, Klefstrom J, Evan GI, Jones N . The oncogenic potential of Kaposi's sarcoma-associated herpesvirus cyclin is exposed by p53 loss in vitro and in vivo. Cancer Cell 2002; 2: 229–241.
Verschuren EW, Hodgson JG, Gray JW, Kogan S, Jones N, Evan GI . The role of p53 in suppression of KSHV cyclin-induced lymphomagenesis. Cancer Res 2004; 64: 581–589.
Clark JC, Dass CR, Choong PF . A review of clinical and molecular prognostic factors in osteosarcoma. J Cancer Res Clin Oncol 2008; 134: 281–297.
Walkley CR, Qudsi R, Sankaran VG, Perry JA, Gostissa M, Roth SI et al. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev 2008; 22: 1662–1676.
Berman SD, Calo E, Landman AS, Danielian PS, Miller ES, West JC et al. Metastatic osteosarcoma induced by inactivation of Rb and p53 in the osteoblast lineage. Proc Natl Acad Sci USA 2008; 105: 11851–11856.
Whelan J, McTiernan A, Cooper N, Wong YK, Francis M, Vernon S et al. Incidence and survival of malignant bone sarcomas in England 1979-2007. Int J Cancer 2011; 131: E508–E517.
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 1997; 89: 755–764.
Inada M, Yasui T, Nomura S, Miyake S, Deguchi K, Himeno M et al. Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev Dyn 1999; 214: 279–290.
Shimomura Y, Yoneda T, Suzuki F . Osteogenesis by chondrocytes from growth cartilage of rat rib. Calcif Tissue Res 1975; 19: 179–187.
Acknowledgements
We thank H Okayama and T Ogasawara for providing DNA plasmids for gene expression, M Hirakawa, M Mori and Y Matsuo for technical assistance, and C Fukuda for secretarial assistance. This work was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and the President’s Discretionary Fund of Nagasaki University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ito, K., Maruyama, Z., Sakai, A. et al. Overexpression of Cdk6 and Ccnd1 in chondrocytes inhibited chondrocyte maturation and caused p53-dependent apoptosis without enhancing proliferation. Oncogene 33, 1862–1871 (2014). https://doi.org/10.1038/onc.2013.130
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.130