Abstract
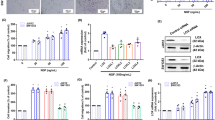
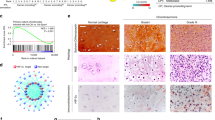
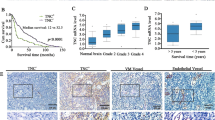
Chondrosarcoma is the second most common sarcoma in bone malignancy and is characterized by a high metastatic potential. Angiogenesis is essential for the cancer metastasis. Endothelin-1 (ET-1) has been implicated in tumor angiogenesis and metastasis. However, the relationship of ET-1 with vascular endothelial growth factor (VEGF) expression and angiogenesis in human chondrosarcoma cells is mostly unknown. Here, we found that the expression of ET-1 and VEGF were correlated with tumor stage and were significantly higher than that in the normal cartilage. Exogenous ET-1 with chondrosarcoma cells promoted VEGF expression and subsequently increased migration and tube formation in endothelial progenitor cells. ET-1 increased VEGF expression and angiogenesis through ETAR, integrin-linked kinase (ILK), Akt and hypoxia-inducible factor-1α (HIF-1α) signaling cascades. Knockdown of ET-1 decreased VEGF expression and also abolished chondrosarcoma conditional medium-mediated angiogenesis in vitro as well as angiogenesis effects in the chick chorioallantoic membrane and Matrigel plug nude mice model in vivo. In addition, in the xenograft tumor angiogenesis model, knockdown of ET-1 significantly reduced tumor growth and tumor-associated angiogenesis. Taken together, these results indicate that ET-1 occurs through ETAR, ILK and Akt, which in turn activates HIF-1α, resulting in the activation of VEGF expression and contributing to the angiogenesis and tumor growth of human chondrosarcoma cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Terek RM, Schwartz GK, Devaney K, Glantz L, Mak S, Healey JH et al. Chemotherapy and P-glycoprotein expression in chondrosarcoma. J Orthop Res 1998; 16: 585–590.
Bishayee A, Darvesh AS . Angiogenesis in hepatocellular carcinoma: a potential target for chemoprevention and therapy. Curr Cancer Drug Targets 2012; 12: 1095–1118.
Albini A, Tosetti F, Li VW, Noonan DM, Li WW . Cancer prevention by targeting angiogenesis. Nat Rev Clin Oncol 2012; 9: 498–509.
Weis SM, Cheresh DA . Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med 2011; 17: 1359–1370.
Keating AM, Jacobs DS . Anti-VEGF treatment of corneal neovascularization. Ocul Surf 2011; 9: 227–237.
Herrmann E, Bogemann M, Bierer S, Eltze E, Hertle L, Wulfing C . The endothelin axis in urologic tumors: mechanisms of tumor biology and therapeutic implications. Expert Rev Anticancer Ther 2006; 6: 73–81.
Wulfing P, Kersting C, Tio J, Fischer RJ, Wulfing C, Poremba C et al. Endothelin-1-, endothelin-A-, and endothelin-B-receptor expression is correlated with vascular endothelial growth factor expression and angiogenesis in breast cancer. Clin Cancer Res 2004; 10: 2393–2400.
Lahav R, Suva ML, Rimoldi D, Patterson PH, Stamenkovic I . Endothelin receptor B inhibition triggers apoptosis and enhances angiogenesis in melanomas. Cancer Res 2004; 64: 8945–8953.
Lamagna C, Aurrand-Lions M, Imhof BA . Dual role of macrophages in tumor growth and angiogenesis. J Leukocyte Biol 2006; 80: 705–713.
Spinella F, Rosano L, Del Duca M, Di Castro V, Nicotra MR, Natali PG et al. Endothelin-1 inhibits prolyl hydroxylase domain 2 to activate hypoxia-inducible factor-1alpha in melanoma cells. PLoS One 2010; 5: e11241.
Boldrini L, Pistolesi S, Gisfredi S, Ursino S, Ali G, Pieracci N et al. Expression of endothelin 1 and its angiogenic role in meningiomas. Virchows Archiv 2006; 449: 546–553.
Zhou WQ, Yin HL, Zhang ZY, Yi XM, Ge JP, Zhou SG et al. [Expression of VEGF in prostate cancer and its correlation with ET-1]. Natl J Androl 2008; 14: 987–992.
Wu MH, Lo JF, Kuo CH, Lin JA, Lin YM, Chen LM et al. Endothelin-1 promotes MMP-13 production and migration in human chondrosarcoma cells through FAK/PI3K/Akt/mTOR pathways. J Cell Physiol 2012; 227: 3016–3026.
Venuti A, Salani D, Cirilli A, Simeone P, Muller A et al. Endothelin receptor blockade inhibits the growth of human papillomavirus-associated cervical carcinoma. Clin Sci (Lond) 2002; 103 (Suppl 48): 310S–313SS.
Nagy JA, Dvorak AM, Dvorak HF . VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol 2007; 2: 251–275.
Bergers G, Benjamin LE . Tumorigenesis and the angiogenic switch. Nat Rev Cancer 2003; 3: 401–410.
Villers A, Neuzillet Y, Beuzeboc P . Inhibitors of the receptor A for endothelin-1. Progr Urol 2010; 20 (Suppl 1): S77–S79.
Merino M, Pinto A, Gonzalez R, Espinosa E . Antiangiogenic agents and endothelin antagonists in advanced castration resistant prostate cancer. Eur J Cancer 2011; 47: 1846–1851.
Kaneko Y, Kitazato K, Basaki Y . Integrin-linked kinase regulates vascular morphogenesis induced by vascular endothelial growth factor. J Cell Sci 2004; 117 (Part 3): 407–415.
Camenisch G, Stroka DM, Gassmann M, Wenger RH . Attenuation of HIF-1 DNA-binding activity limits hypoxia-inducible endothelin-1 expression. Pflugers Arch 2001; 443: 240–249.
Whitman EM, Pisarcik S, Luke T, Fallon M, Wang J, Sylvester JT et al. Endothelin-1 mediates hypoxia-induced inhibition of voltage-gated K+ channel expression in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 2008; 294: L309–L318.
Yen ML, Su JL, Chien CL, Tseng KW, Yang CY, Chen WF et al. Diosgenin induces hypoxia-inducible factor-1 activation and angiogenesis through estrogen receptor-related phosphatidylinositol 3-kinase/Akt and p38 mitogen-activated protein kinase pathways in osteoblasts. Mol Pharmacol 2005; 68: 1061–1073.
Bagnato A, Spinella F, Rosano L . The endothelin axis in cancer: the promise and the challenges of molecularly targeted therapy. Canad J Physiol Pharmacol 2008; 86: 473–484.
Demunter A, De Wolf-Peeters C, Degreef H, Stas M, van den Oord JJ . Expression of the endothelin-B receptor in pigment cell lesions of the skin. Evidence for its role as tumor progression marker in malignant melanoma. Virchows Archiv 2001; 438: 485–491.
Spinella F, Rosano L, Di Castro V, Natali PG, Bagnato A . Endothelin-1-induced prostaglandin E2-EP2, EP4 signaling regulates vascular endothelial growth factor production and ovarian carcinoma cell invasion. J Biol Chem 2004; 279: 46700–46705.
Bagnato A, Spinella F . Emerging role of endothelin-1 in tumor angiogenesis. Trends Endocrinol Metab 2003; 14: 44–50.
Lahav R, Heffner G, Patterson PH . An endothelin receptor B antagonist inhibits growth and induces cell death in human melanoma cells in vitro and in vivo. Proc Natl Acad Sci USA 1999; 96: 11496–11500.
Zhang H, Han Y, Tao J, Liu S, Yan C, Li S . Cellular repressor of E1A-stimulated genes regulates vascular endothelial cell migration by the ILK/AKT/mTOR/VEGF(165) signaling pathway. Exp Cell Res 2011; 317: 2904–2913.
Tseng WP, Yang SN, Lai CH, Tang CH . Hypoxia induces BMP-2 expression via ILK, Akt, mTOR, and HIF-1 pathways in osteoblasts. J Cell Physiol 2010; 223: 810–818.
Rosano L, Spinella F, Di Castro V, Dedhar S, Nicotra MR, Natali PG et al. Integrin-linked kinase functions as a downstream mediator of endothelin-1 to promote invasive behavior in ovarian carcinoma. Mol Cancer Therap 2006; 5: 833–842.
Cianfrocca R, Rosano L, Spinella F, Di Castro V, Natali PG, Bagnato A . Beta-arrestin-1 mediates the endothelin-1-induced activation of Akt and integrin-linked kinase. Canad J Physiol Pharmacol 2010; 88: 796–801.
Wang HH, Lin CA, Lee CH, Lin YC, Tseng YM, Hsieh CL et al. Fluorescent gold nanoclusters as a biocompatible marker for in vitro and in vivo tracking of endothelial cells. ACS Nano 2011; 5: 4337–4344.
Hsieh MT, Hsieh CL, Lin LW, Wu CR, Huang GS . Differential gene expression of scopolamine-treated rat hippocampus-application of cDNA microarray technology. Life Sci 2003; 73: 1007–1016.
Wang YC, Lee PJ, Shih CM, Chen HY, Lee CC, Chang YY et al. Damage formation and repair efficiency in the p53 gene of cell lines and blood lymphocytes assayed by multiplex long quantitative polymerase chain reaction. Anal Biochem 2003; 319: 206–215.
Huang HC, Shi GY, Jiang SJ, Shi CS, Wu CM, Yang HY et al. Thrombomodulin-mediated cell adhesion: involvement of its lectin-like domain. J Biol Chem 2003; 278: 46750–46759.
Tseng CP, Huang CL, Huang CH, Cheng JC, Stern A, Tseng CH et al. Disabled-2 small interfering RNA modulates cellular adhesive function and MAPK activity during megakaryocytic differentiation of K562 cells. FEBS Lett 2003; 541: 21–27.
Tang CH, Lu DY, Tan TW, Fu WM, Yang RS . Ultrasound induces hypoxia-inducible factor-1 activation and inducible nitric-oxide synthase expression through the integrin/integrin-linked kinase/Akt/mammalian target of rapamycin pathway in osteoblasts. J Biol Chem 2007; 282: 25406–25415.
Xia X, Lemieux ME, Li W, Carroll JS, Brown M, Liu XS et al. Integrative analysis of HIF binding and transactivation reveals its role in maintaining histone methylation homeostasis. Proc Natl Acad Sci USA 2009; 106: 4260–4265.
Nickols NG, Jacobs CS, Farkas ME, Dervan PB . Modulating hypoxia-inducible transcription by disrupting the HIF-1–DNA interface. ACS Chem Biol 2007; 2: 561–571.
Storgard C, Mikolon D, Stupack DG . Angiogenesis assays in the chick CAM. Methods Mol Biol 2005; 294: 123–136.
Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, Haney JA et al. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest 1992; 67: 519–528.
Acknowledgements
We thank Dr WM Fu for providing Akt mutant and HRE promoter construct. This work was supported by grants from the National Science Council of Taiwan (NSC99-2320-B-039-003-MY3 and NSC100-2320-B-039-028-MY3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Wu, MH., Huang, CY., Lin, JA. et al. Endothelin-1 promotes vascular endothelial growth factor-dependent angiogenesis in human chondrosarcoma cells. Oncogene 33, 1725–1735 (2014). https://doi.org/10.1038/onc.2013.109
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.109
Keywords
This article is cited by
-
Outgrowth Endothelial Cell Conditioned Medium Negates TNF-α-Evoked Cerebral Barrier Damage: A Reverse Translational Research to Explore Mechanisms
Stem Cell Reviews and Reports (2023)
-
Quercetin ameliorates acute lung injury in a rat model of hepatopulmonary syndrome
BMC Complementary Medicine and Therapies (2022)
-
Genome wide effects of oleic acid on cultured bovine granulosa cells: evidence for the activation of pathways favoring folliculo-luteal transition
BMC Genomics (2021)
-
CXCL13/CXCR5 axis facilitates endothelial progenitor cell homing and angiogenesis during rheumatoid arthritis progression
Cell Death & Disease (2021)
-
Retinal venous pressure is decreased after anti-VEGF therapy in patients with retinal vein occlusion–related macular edema
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)