Abstract
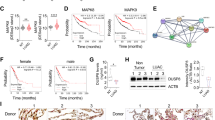
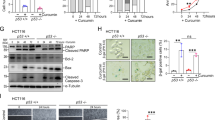
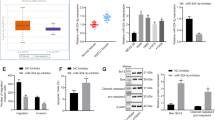
Resveratrol, a phytochemical found in various plants and Chinese herbs, is associated with multiple tumor-suppressing activities, has been tested in clinical trials. However, the molecular mechanisms involved in resveratrol-mediated tumor suppressing activities are not yet completely defined. Here, we showed that treatment with resveratrol inhibited cell mobility through induction of the mesenchymal–epithelial transition (MET) in lung cancer cells. We also found that downregulation of FOXC2 (forkhead box C2) is critical for resveratrol-mediated suppression of tumor metastasis in an in vitro and in vivo models. We also identified a signal cascade, namely, resveratrol—∣miRNA-520h—∣PP2A/C—∣Akt → NF-κB → FOXC2, in which resveratrol inhibited the expression of FOXC2 through regulation of miRNA-520h-mediated signal cascade. This study identified a new miRNA-520h-related signal cascade involved in resveratrol-mediated tumor suppression activity and provide the clinical significances of miR-520h, PP2A/C and FOXC2 in lung cancer patients. Our results indicated a functional link between resveratrol-mediated miRNA-520h regulation and tumor suppressing ability, and provide a new insight into the role of resveratrol-induced molecular and epigenetic regulations in tumor suppression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Paget S . The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 1989; 8: 98–101.
Polyak K, Weinberg RA . Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 2009; 9: 265–273.
Kim JB, Islam S, Kim YJ, Prudoff RS, Sass KM, Wheelock MJ et al. N-Cadherin extracellular repeat 4 mediates epithelial to mesenchymal transition and increased motility. J Cell Biol 2000; 151: 1193–1206.
Ngan CY, Yamamoto H, Seshimo I, Tsujino T, Man-i M, Ikeda JI et al. Quantitative evaluation of vimentin expression in tumour stroma of colorectal cancer. Br J Cancer 2007; 96: 986–992.
Kowalski PJ, Rubin MA, Kleer CG . E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res 2003; 5: 217–222.
Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP . Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J 2004; 23: 1155–1165.
Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev 2004; 18: 99–115.
Barrallo-Gimeno A, Nieto MA . The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 2005; 14: 3151–3161.
Alves CC, Carneiro F, Hoefler H, Becker KF . Role of the epithelial-mesenchymal transition regulator Slug in primary human cancers. Front Biosci 2009; 14: 3035–3050.
Browne G, Sayan AE, Tulchinsky E . ZEB proteins link cell motility with cell cycle control and cell survival in cancer. Cell Cycle 2010; 9: 886–991.
Hamamori Y, Wu HY, Sartorelli V, Kedes L . The basic domain of myogenic basic helix-loop-helix (bHLH) proteins is the novel target for direct inhibition by another bHLH protein, Twist. Mol Cell Biol 1997; 17: 6563–6573.
Hartwell KA, Muir B, Reinhardt F, Carpenter AE, Sgroi DC, Weinberg RA . The Spemann organizer gene, Goosecoid, promotes tumor metastasis. Proc Natl Acad Sci USA 2006; 103: 18969–18974.
Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N et al. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci USA 2007; 104: 10069–10074.
Hader C, Marlier A, Cantley L . Mesenchymal-epithelial transition in epithelial response to injury: the role of Foxc2. Oncogene 2010; 29: 1031–1040.
Nishida N, Mimori K, Yokobori T, Sudo T, Tanaka F, Shibata K et al. FOXC2 is a Novel Prognostic Factor in Human Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 2011; 2: 535–542.
Baur JA, Sinclair DA . Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 2006; 5: 493–506.
Saiko P, Szakmary A, Jaeger W, Szekeres T . Resveratrol and its analogs: defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat Res 2008; 658: 68–94.
Holmes-McNary M, Baldwin Jr AS . Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res 2000; 60: 3477–3483.
Choi HK, Yang JW, Kang KW . Bifunctional effect of resveratrol on the expression of ErbB2 in human breast cancer cell. Cancer Let 2006; 242: 198–206.
Li Q, Li J, Ren J . UCF-101 mitigates streptozotocin-induced cardiomyocyte dysfunction: role of AMPK. Am J Physiol Endocrinol Metab 2009; 4: 965–973.
Woo JH, Lim JH, Kim YH, Suh SI, Min DS, Chang JS et al. Resveratrol inhibits phorbol myristate acetate-induced matrix metalloproteinase-9 expression by inhibiting JNK and PKC delta signal transduction. Oncogene 2004; 23: 1845–1853.
Aziz MH, Nihal M, Fu VX, Jarrard DF, Ahmad N . Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3′-kinase/Akt pathway and Bcl-2 family proteins. Mol Cancer Ther 2006; 5: 1335–1341.
Stewart JR, Ward NE, Ioannides CG, O’Brian CA . Resveratrol preferentially inhibits protein kinase C-catalyzed phosphorylation of a cofactor-independent, arginine-rich protein substrate by a novel mechanism. Biochemistry 1999; 38: 13244–13251.
Stewart JR, Christman KL, O’Brian CA . Effects of resveratrol on the autophosphorylation of phorbol ester-responsive protein kinases: inhibition of protein kinase D but not protein kinase C isozyme autophosphorylation. Biochem Pharmacol 2000; 60: 1355–1359.
Heinemeyer T, Wingender E, Reuter I, Hermjakob H, Kel A, Kel O et al. Databases on transcriptional regulation: TRANSFAC, TRRD, and COMPEL. Nucleic Acids Res 1998; 26: 364–370.
Aggarwal BB . Nuclear factor-κB:The enemy within. Cancer Cell 2004; 6: 203–208.
Gronning LM, Cederberg A, Miura N, Enerback S, Tasken K . Insulin and TNF alpha induce expression of the forkhead transcription factor gene Foxc2 in 3T3-L1 adipocytes via PI3K and ERK 1/2-dependent pathways. Mol Endocrinol 2002; 16: 873–883.
Hayashi H, Kume T . Foxc transcription factors directly regulate Dll4 and Hey2 expression by interacting with the VEGF-Notch signaling pathways in endothelial cells. PLoS One 2008; 3: e2401.
Sun HZ, Yang TW, Zang WJ, Wu SF . Dehydroepiandrosterone-induced proliferation of prostatic epithelial cell is mediated by NFKB via PI3K/AKT signaling pathway. Eur J Endocrinol/Eur Fed Endocr Soc 2010; 204: 311–318.
Liao Y HM . A new role of protein phosphatase 2a in adenoviral E1A protein-mediated sensitization to anticancer drug-induced apoptosis in human breast cancer cells. Cancer Res 2004; 64: 5938–5942.
Garzon R, Marcucci G, Croce CM . Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov 2010; 9: 775–789.
Su JL, Chen PB, Chen YH, Chen SC, Chang YW, Jan YH et al. Downregulation of microRNA miR-520h by E1A contributes to anticancer activity. Cancer Res 2010; 70: 5096–5108.
Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL . Multiple molecular targets of resveratrol: anti-carcinogenic mechanisms. Arch Biochem Biophys 2009; 486: 95–102.
Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y . Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res 2004; 24: 2783–2840.
Kume T, Jiang H, Topczewska JM, Hogan BL . The murine winged helix transcription factors, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis. Genes Dev 2001; 15: 2470–2482.
Sano H, Leboeuf JP, Novitskiy SV, Seo S, Zaja-Milatovic S, Dikov MM et al. The Foxc2 transcription factor regulates tumor angiogenesis. Biochem Biophys Res Commun 2010; 392: 201–206.
Topczewska JM, Topczewski J, Shostak A, Kume T, Solnica-Krezel L, Hogan BL . The winged helix transcription factor Foxc1a is essential for somitogenesis in zebrafish. Genes Dev 2001; 15: 2483–2493.
Singh A, Settleman J . EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 2010; 29: 4741–4751.
Larue L, Bellacosa A . Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene 2005; 24: 7443–7454.
Lu Y, Wahl LM . Production of matrix metalloproteinase-9 by activated human monocytes involves a phosphatidylinositol-3 kinase/Akt/IKKalpha/NF-kappaB pathway. J Leukoc Biol 2005; 78: 259–265.
Agarwal A, Das K, Lerner N, Sathe S, Cicek M, Casey G et al. The AKT/I kappa B kinase pathway promotes angiogenic/metastatic gene expression in colorectal cancer by activating nuclear factor-kappa B and beta-catenin. Oncogene 2005; 24: 1021–1031.
Julien S, Puig I, Caretti E, Bonaventure J, Nelles L, van Roy F et al. Activation of NF-kappaB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene 2007; 26: 7445–7456.
Millward TA, Zolnierowicz S, Hemmings BA . Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci 1999; 24: 186–191.
Perrotti D, Neviani P . Protein phosphatase 2A (PP2A), a drugable tumor suppressor in Ph1(+) leukemias. Cancer Metastasis Rev 2008; 27: 159–168.
Neviani P, Santhanam R, Trotta R, Notari M, Blaser BW, Liu S et al. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell 2005; 8: 355–368.
Feschenko MS, Stevenson E, Nairn AC, Sweadner KJ . A novel cAMP-stimulated pathway in protein phosphatase 2A activation. J Pharmacol Exp Ther 2002; 302: 111–118.
Matsuoka Y, Nagahara Y, Ikekita M, Shinomiya T . A novel immunosuppressive agent FTY720 induced Akt dephosphorylation in leukemia cells. Br J Pharmacol 2003; 138: 1303–1312.
Esquela-Kerscher A, Slack FJ . Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer 2006; 6: 259–269.
Korpal M, Kang Y . The emerging role of miR-200 family of microRNAs in epithelial-mesenchymal transition and cancer metastasis. RNA Biol 2008; 5: 115–119.
Tili E, Michaille JJ, Adair B, Alder H, Limagne E, Taccioli C et al. Resveratrol decreases the levels of miR-155 by upregulating miR-663, a microRNA targeting JunB and JunD. Carcinogenesis 2010; 31: 1561–1566.
Tili E, Michaille JJ, Alder H, Volinia S, Delmas D, Latruffe N et al. Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFbeta signaling pathway in SW480 cells. Biochem Pharmacol 2010; 80: 2057–2065.
Huang Q, Gumireddy K, Schrier M, le Sage C, Nagel R, Nair S et al. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol 2008; 10: 202–210.
Chen MW, Hua KT, Kao HJ, Chi CC, Wei LH, Johansson G et al. H3K9 histone methyltransferase G9a promotes lung cancer invasion and metastasis by silencing the cell adhesion molecule Ep-CAM. Cancer Res 2010; 70: 7830–7840.
Kuhn H, Liebers U, Gessner C, Schumacher A, Witt C, Schauer J et al. Adenorivus-mediated E2F-1 gene transfer in nonsmall-cell lung cancer induces cell growth arrest and apoptosis. Eur Respir J 2002; 20: 703–709.
Acknowledgements
This work was partially supported by the National Science Council Grant (NSC-2632-B-001-MY3, NSC 96-2320-B-004-MY2, NSC 97-2320-B-039-039-MY3 and NSC 98-2815-C-039-082-B to J-LS); National Health Research Institutes Grant from Taiwan (NHRI-EX98-9712BC, NHRI-EX99-9712BC and NHRI-EX100-9712BC to J-LS); Department of Health, Executive Yuan Grant from Taiwan (DOH99-TD-G111-011 to J-LS); Grants from China Medical University (CMU96-220, CMU97-077 and CMU97-277 to J-LS; CMU-99-NTU-08, CMU100-TS-06 and DMR-101-014 to Y-HY).
Author contributions: J-LS, M-HC, M-CH and M-LK designed and conceived the study. P-SC and J-LS wrote the manuscript. Y-WC, Y-HC, H-AC and Y-JC performed the experiments. C-HW, M-TH, H-AC, Y-HS, W-HH, Y-MJ, C-HH, Y-HJ and MH performed and analyzed the immunohistochemistry experiments.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Yu, YH., Chen, HA., Chen, PS. et al. MiR-520h-mediated FOXC2 regulation is critical for inhibition of lung cancer progression by resveratrol. Oncogene 32, 431–443 (2013). https://doi.org/10.1038/onc.2012.74
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.74
Keywords
This article is cited by
-
Homology modeling of Forkhead box protein C2: identification of potential inhibitors using ligand and structure-based virtual screening
Molecular Diversity (2023)
-
Dysregulated ceramides metabolism by fatty acid 2-hydroxylase exposes a metabolic vulnerability to target cancer metastasis
Signal Transduction and Targeted Therapy (2022)
-
Blocking MMP-12-modulated epithelial-mesenchymal transition by repurposing penfluridol restrains lung adenocarcinoma metastasis via uPA/uPAR/TGF-β/Akt pathway
Cellular Oncology (2021)
-
Stabilization of ADAM9 by N-α-acetyltransferase 10 protein contributes to promoting progression of androgen-independent prostate cancer
Cell Death & Disease (2020)
-
Resveratrol (3, 5, 4′-Trihydroxy-trans-Stilbene) Attenuates a Mouse Model of Multiple Sclerosis by Altering the miR-124/Sphingosine Kinase 1 Axis in Encephalitogenic T Cells in the Brain
Journal of Neuroimmune Pharmacology (2019)