Abstract
GRP78, a major endoplasmic reticulum chaperone and signaling regulator, is commonly overexpressed in cancer. Moreover, induction of GRP78 by a variety of anti-cancer drugs, including histone deacetylase inhibitors, confers chemoresistance to cancer, thereby contributing to tumorigenesis. Thus, therapies aimed at decreasing GRP78 levels, which results in the inhibition of tumor cell proliferation and resensitization of tumor cells to chemotherapeutic drugs may hold promise for cancer treatment. Despite advances in our understanding of GRP78 actions, little is known about endogenous inhibitors controlling its expression. As endogenous regulators, microRNAs (miRNAs) play important roles in modulating gene expression; therefore, we sought to identify miRNA(s) that target GRP78, under the hypothesis that these miRNAs may serve as therapeutic agents. Here, we report that three miRNAs (miR-30d, miR-181a, miR-199a-5p) predicted to target GRP78 are down-regulated in prostate, colon and bladder tumors, and human cancer cell lines. We show that in C42B prostate cancer cells, these miRNAs down-regulate GRP78 and induce apoptosis by directly targeting its 3' untranslated region. Importantly, we demonstrate that the three miRNAs act cooperatively to decrease GRP78 levels, suggesting that multiple miRNAs may be required to efficiently control the expression of some genes. In addition, delivery of multiple miRNAs by either transient transfection or lentivirus transduction increased the sensitivity of cancer cells to the histone deacetylase inhibitor, trichostatin A, in C42B, HCT116 and HL-60 cells. Together, our results indicate that the delivery of co-transcribed miRNAs can efficiently suppress GRP78 levels and GRP78-mediated chemoresistance, and suggest that this strategy holds therapeutic potential.
Similar content being viewed by others
Introduction
The glucose regulated protein GRP78, also referred to as BiP or HSPA5, is a major endoplasmic reticulum (ER) chaperone and a master regulator of the unfolded protein response (UPR).1, 2, 3, 4 GRP78 can also be detected on the surface of cancer cells, where it mediates oncogenic signals.5 The stress-mediated up-regulation of GRP78 represents a key adaptive response for cancer cell survival.1 However, under severe stress conditions, apoptosis is triggered through the induction of BAX/BAK and CHOP.3, 6 Tumors are subjected to ER stress due to intrinsic metabolic alterations and extrinsic factors in the tumor microenvironment, such as acidosis and hypoxia, which leads to UPR activation and GRP78 induction.7, 8 GRP78 is up-regulated in a variety of tumors, and plays roles in anti-apoptosis, tumor progression, angiogenesis and metastasis.1, 8, 9, 10, 11 Increased GRP78 levels are associated with poor outcome and early recurrence in prostate cancer.12 Conversely, GRP78 haploinsufficiency in mouse cancer models slows the progression of both solid tumors and hematopoietic malignancies, and inhibits tumor neo-angiogenesis.7, 13, 14 Increased GRP78 expression in cancer cells facilitates drug resistance by suppressing apoptosis,1, 15 whereas knockdown of GRP78 sensitizes human cancer cells to the histone deacetylase inhibitor trichostatin A (TSA), malignant gliomas to temozolomide and tumor-associated endothelial cells to chemotherapeutic agents.16, 17, 18 Collectively, these studies suggest that GRP78 is a potential therapeutic target in cancer.1, 19
Small regulatory RNAs, such as microRNAs (miRNAs), endogenously suppress gene expression,20 and hundreds of miRNAs have been described.21 Human miRNAs regulate several important biological processes, including cell proliferation and differentiation, development, apoptosis and epigenetic changes,22, 23 and are implicated in diseases, such as cancer.24, 25, 26 Aberrant expression of miRNAs is common in various cancers and can be caused by genomic abnormalities, miRNA processing defects and epigenetic alterations.27, 28, 29, 30, 31 MiRNAs can act as oncogenes or as tumor suppressors by targeting molecules critically involved in carcinogenesis24, 32, 33 and thus, they are good candidate targets for cancer therapy.27, 34 In addition, miRNAs have been implicated in the stress response.35, 36 Since increased GRP78 expression is involved in tumor progression and chemoresistance, we hypothesized that miRNAs that target GRP78, which is highly expressed in cancer, may act as tumor suppressors and may be clinically relevant. To date, however, the specific miRNAs involved in the regulation of GRP78 have yet to be characterized.
Here, we show that miR-30d, miR-181a and miR-199a-5p, three miRNAs predicted to target GRP78 in silico, are down-regulated in tumors of various origins and in human cancer cell lines. Further, we demonstrate an inverse correlation between miRNA and GRP78 expression levels, suggesting that these miRNAs may regulate GRP78 and are clinically relevant. Our results indicate that the three miRNAs directly bind to and down-regulate GRP78 levels in vitro. In cancer cells, miRNAs showed combinatorial effects on GRP78 repression, and apoptosis induction as well as on the reduction of cell survival and colony formation. Importantly, the cooperation among these miRNAs increased the sensitivity of cancer cells to drug treatment. Altogether, our results identify three specific miRNAs that regulate GPR78 and provide evidence that the delivery of multiple miRNAs holds promise for the development of novel cancer therapies.
Results
Inverse relationship between the expression of miR-30d, miR-181a and miR-199a-5p and their putative target GRP78
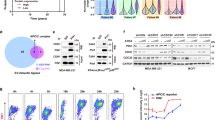
In order to identify the specific miRNAs that target the 3′ untranslated region (3′UTR) of GRP78, we used the TargetScan database (http://www.targetscan.org). Of all predicted miRNAs, we selected three—miR-30d, miR-181a and miR-199a-5p—that have highly conserved seed sequences at the 3′UTR of GRP78 (Figure 1a; Supplementary Figure S1). We first explore whether the correlation of these three miRNAs and GRP78 could be clinical relevant by measuring the expression of these three miRNAs and GRP78 mRNA levels in normal-tumor paired samples from patients with prostate, colon and bladder cancer. We found an inverse correlation between miRNAs and GRP78 in all three types of tumors (P<0.05) (Figure 1b). In order to find optimal subjects for in vitro studies, we next examined the endogenous expression levels of these miRNAs in non-tumorigenic (LD419, UROtsa and NK2464) and tumorigenic cell lines (lymphoma, lung, cervical, bladder, colon and prostate). Differential expression of the three miRNAs was observed in cell lines (Figure 1c). In general, miR-30d and miR-181a were down-regulated in most cancer cell lines and tumor samples, whereas miR-199a-5p maintained low expression levels in most normal and cancer cell lines, with the exception of LD419. In contrast, clinical samples showed a clear decrease in miR-199a-5p expression compared with normal paired controls (Figure 1b).
Relative expression of miR-30d, miR-181a and miR-199a-5p, and their potential target, GRP78, in human cell lines. (a) Prediction of putative miRNAs targeting human GRP78 using TargetScan (http://www.targetscan.org). The positions of miR-30d, miR-181a and miR-199a-5p base paring with the 3′UTR of GRP78 are indicated. (b) miR-30d, miR-181a and miR-199a-5p and GRP78 expression in normal-tumor paired samples from patients with prostate, colon and bladder cancer. Paired t-test was performed. *P<0.05. (c) Endogenous expression of miR-30d, miR-181a and miR-199a-5p was determined by quantitative real-time PCR analysis in two non-tumorigenic and four tumorigenic human cell lines (indicated with dashed lines) and normalized to U6 snRNA. (d) GRP78 protein levels were assessed by western blot in untreated cell lines (e) and in cells treated with the ER stressor Tg, quantitated using Quantity One (Bio-Rad), and plotted as a ratio to Actin levels. *P<0.05; **P<0.01; ***P<0.001.
Next, we examined the correlation between the expression levels of miR-30d, miR-181a and miR-199a-5p and GRP78 protein levels. We selected a panel of two non-tumorigenic cell lines (LD419 and UROtsa), which have high expression levels of all three miRNAs (except miR-199a-5p in UROtsa), and four cancer cell lines, which displayed both high (UM-UC-3, J82) and low (HCT116 and C42B) miRNA levels. Based on the miRNA expression profile, we found that GRP78 expression was higher in HCT116 and C42B, and quite low in other cell lines (Figure 1d), suggesting a regulatory role of these miRNAs on GRP78 expression.
GRP78 expression is induced in cells following exposure to the ER stress inducer, thapsigargin (Tg).16, 17 To determine how the endogenous expression levels of these miRNAs affected the cell response to Tg, we examined GRP78 protein levels 20 h after Tg treatment. Treatment with Tg caused a fourfold increase in GRP78 protein levels compared with untreated cells. We found that GRP78 induction was the highest in HCT116 and C42B cells, which lack the expression of all three miRNAs (Figure 1e). In addition, GRP78 levels were significantly different between cells with up-regulated expression of the three miRNAs (LD419, UROtsa, UMUC3 and J82), and cells with low expression of the three miRNAs (HCT116 and C42B), and these differences persisted even under conditions known to induce GRP78, such as Tg treatment (P<0.05, n=3). Our results also demonstrate a similar pattern of GRP78 expression at the mRNA level (Supplementary Figure S1B). Collectively, these studies show that both basal and Tg-induced GRP78 levels inversely associate with the endogenous levels of the three miRNAs analyzed.
MiR-30d, miR-181a and miR-199a-5p directly target the 3′UTR of GRP78 and significantly suppress luciferase activity cooperatively
To investigate whether GRP78 is a direct target of miR-30d, miR-181a and miR-199a-5p, we generated a firefly luciferase reporter vector containing the GRP78 3′UTR (Figure 2a). The vector was transfected along with miRNA precursors into C42B cells, since these cells lack the expression of all three miRNAs. The lysates were analyzed for luciferase activity 24 h post-transfection. We found mild repression of luciferase activity by individual miRNAs, which did not reach statistical significance, whereas the transfection with all three miRNAs resulted in approximately a 60% decrease in activity, suggesting that they act cooperatively (P<0.05, n=3) (Figure 2a). However, the combination of any given two miRNAs may also have a similar effect. To assess this possibility, we generated mutant GRP78 luciferase constructs, carrying two base pair changes in each miRNA putative binding site located at the GRP78 3′UTR (Figure 2b). All three miRNA precursors were transfected along with wild-type (WT) or mutated GRP78 vectors into C42B cells to test the binding ability of different miRNA combinations. Mutation in any of the three miRNA-binding sequences reduced the ability of all three miRNAs to inhibit luciferase activity, suggesting that they directly bind to the 3′UTR of GRP78 and all three are required to achieve efficient inhibition.
miR-30d, miR-181a and miR-199a-5p directly target the 3′UTR of GRP78 with cooperative effects. (a) The luciferase reporter vector (pGL3) containing the human GRP78 3′UTR was co-transfected with miR-30d, miR-181a or miR-199a-5p precursors into C42B cells at a final concentration of 150 nM. Luciferase activity was measured 24 h after transfection and normalized to Renilla (pRL-SV40). (b) Individual miRNA-binding sites (Luc-m1, Luc-m2 and Luc-m3) or all three miRNA-binding sites (Luc-m123) were mutated in the GRP78 3′UTR vector. Luciferase reporter vectors, either WT or mutant (m1, m2, m3 and m123) were co-transfected with either miRNC or all three miRNA precursors into C42B cells at a final concentration of 150 nM. Luciferase activity was measured 24 h after transfection and normalized to Renilla (pRL-SV40). Data are shown as the mean±s.d. of three individual experiments. *P<0.05. miRNC: negative control.
GRP78 down-regulation requires cooperation of multiple miRNAs and leads to morphological changes and apoptosis in C42B cells
GRP78 is a stable protein present in cancer cell lines and miRNAs target mRNAs at their 3′UTR region. To further verify the ability of miR-30d, miR-181a and miR-199a-5p to down-regulate GRP78, C42B cells were transfected with miRNA precursors and treated 24 h after transfection with Tg to induce GRP78 expression. MiRNA expression and GRP78 mRNA levels were confirmed by quantitative PCR analysis 20 h post-treatment, an optimal time point for ER chaperon induction, including GRP78, in C42B cells (data not shown). As expected, GRP78 mRNA and protein levels significantly increased after Tg treatment in the control samples (miRNC) (P<0.05, n=3) (Figures 3a and b). A small, non-significant decrease in GRP78 mRNA levels was found in cells treated with Tg and transfected with each individual miRNAs, compared with controls (miRNC). In contrast, when the three miRNAs were transfected together, we observed a significant decrease in GRP78 mRNA levels (P<0.05, n=3) (Figure 3a), which is consistent with the findings of the luciferase experiments described above (Figure 2a). Similarly, a 50% reduction in GRP78 protein levels was observed only when the three miRNAs were transfected together, suggesting again that they act cooperatively (Figure 3b).
Inhibition of GRP78 levels and induction of apoptosis in C42B cells by co-expression of multiple miRNAs. (a) GRP78 mRNA and (b) protein levels were determined by quantitative real-time PCR and western blot, respectively, in cells transfected with miRNA precursors at a final concentration of 50 nM. GRP78 mRNA expression was normalized to human GAPDH and protein levels were normalized to Actin. Data are shown as the mean±s.d. (n=3). *P<0.05. miRNC: negative control. (c) Multiple miRNA expression induced a decrease in GRP78 protein levels and increases in the levels of CHOP, a UPR indicator, and the apoptosis indicator PARP-1. Protein levels were normalized to Actin. Data are shown as the mean±s.d. (n=3). *P<0.05. (d) Representative micrographs showing apoptotic cells (arrowhead) in cells transfected with all three miRNAs with or without Tg treatment. (e) Total cell numbers were counted 4 days post-transfection. Data represent mean±s.d. (n=3). *P<0.05. (f) Attached and floating cells were both collected at the indicated time points and stained with Annexin V-FITC and propidium iodide (PI). The percentage of Annexin V-FITC positive (early apoptotic) cells was determined by FACS. Data are shown as the mean±s.d. (n=3). *P<0.05; **P<0.001; ***P<0.00001.
Since overexpression of GRP78 in cancer cells can inhibit apoptosis,1, 9, 37 we next evaluated whether the modulation of GRP78 levels by miRNAs affects apoptosis in C42B cells treated with Tg. In control samples, which show high GRP78 levels, the protein levels of the apoptotic marker PARP-1, and CHOP, a UPR target that is induced in response to Tg, were low. In contrast, when GRP78 levels were decreased by transfection of miR-30d, miR-181a and miR-199a-5p, PARP-1 and CHOP levels were significantly up-regulated (P<0.05, n=3) (Figure 3c). In addition, C42B cells transfected with miRNAs underwent morphological changes and became rounded. Treatment with Tg resulted in the formation of small vesicles that resemble apoptotic bodies and a decrease in cell numbers (Figures 3d and e). We further confirmed the induction of apoptosis by FACS, using Annexin V and propidium iodide staining. The results showed a significant increase (P<0.05, n=3) in apoptosis in cells transfected with all three miRNAs, but not with miRNC at 24, 48 and 72 h post-transfection. Treatment with Tg significantly increased the apoptotic response in cells transfected with all three miRNAs, compared with miRNC (38.2%±2.1 vs 12.8%±1.6 at 48 h; 60.8%±1.3 vs 33.9%±2.8 at 72 h) (P<0.05, n=3) (Figure 3f). Altogether, the results show that a combination of miRNAs efficiently sensitizes C42B cells to apoptosis by reducing GRP78 levels.
MiR-30d, miR-181a, miR-199a-5p increase the sensitivity of cancer cells to the HDAC inhibitor TSA
It has been demonstrated that increased GRP78 expression confers resistance to the HDAC inhibitor TSA, thereby decreasing its therapeutic efficacy.16 Therefore, we next examined whether down-regulation of GRP78 by miRNAs in C24B cells modifies their response to TSA. We found that TSA treatment induced morphological changes in C24B cells transfected with all three miRNAs, but not with control (miRNC) (Figure 4a, upper panel). TSA induced GRP78 protein levels in cells transfected with miRNC, and this increase was suppressed by transfection of miR-30d, miR-181a and miR-199a-5p, which also resulted in increased expression of the apoptotic marker PARP-1 (Figure 4a, lower panel).
Expression of miR-30d, miR-181a and miR-199a-5p increases TSA sensitivity, and reduces cell viability and colony formation in C42B cells. C42B cells were transfected with miR-30d, miR-181a and miR-199a-5p precursors and with a negative (miRNC) and positive (siRNA against GRP78, siGRP78) controls. In all, 24 h post-transfection, C42B cells were treated with vehicle (Ctrl), Tg or the histone deacetylase inhibitor (TSA). (a) Representative micrographs showing changes in cell morphology. GRP78 and PARP-1 protein levels were assessed by western blot analysis. Actin levels were used as loading control. (b) Total cell numbers were counted 4 days post-transfection. Data represent mean±s.d. (n=3). *P<0.05. (c) Colony formation was assessed 14 days after transfection. Data are shown as the mean±s.d. (n=3). *P<0.05.
As shown above, transfection of all three miRNAs caused a significant decrease in cell numbers (Figure 3e), which can also be observed in cells transfected with small interfering RNA (siRNA) against GRP78 (P<0.05, n=3). TSA treatment of cells transfected with miRNC caused a significant decrease in cell numbers (P<0.05, n=3). Importantly, TSA caused further decreases in cell numbers in cells transfected with the three miRNAs (P<0.05, n=3) (Figure 4b). In addition, colony formation assays showed that transfection with the three miRNAs significantly inhibited colony formation in the presence or absence of TSA, in levels comparable to those of cells transfected with siGRP78 (P<0.05, n=3) (Figure 4c). The results indicate that restoring the expression of multiple miRNAs that target GRP78 can sensitize cancer cells to therapeutic epigenetic agents.
Lentiviral delivery of multiple co-transcribed miRNAs decreases GRP78 protein levels and cell viability and induces apoptosis in different cancer cell lines
In order to improve the delivery efficiency of multiple miRNAs and to evaluate the long-term effects of their expression, we generated a lentiviral expression vector containing the combination of miR-30d, miR-181a and miR-199a-5p (Figure 5a), transduced C42B, HCT116 and HL-60 cells, and confirmed expression of each miRNA in transduced cells (Supplementary Figure S2A). Transduction of the lentivector carrying multiple miRNAs caused a significant decrease in GRP78 protein levels and cell viability, and an increase in PARP-1 protein levels in C42B cells treated with Tg and TSA (P<0.05, n=3) (Figures 5b–e), as shown in transient transfections. Tg-treated HCT116 and HL-60 transduced cells showed a decrease and an increase, but not significant in GRP78 and PARP-1 protein levels, respectively, whereas a significant decrease in GRP78 protein levels was observed in TSA-treated HCT116 cells (P<0.05, n=3) (Figures 5c and e). Cell viability was significantly reduced in Tg-treated HL-60 cells and in both HCT116 and HL-60 cells treated with TSA (P<0.05, n=3) (Figure 5d). Finally, Tg-treated HL-60 and HCT116 cells transduced with three miRNAs showed no changes in PARP-1 levels, whereas a non-significant increase in PARP-1 was observed in TSA-treated cells (Figure 5e). In addition, decreased colony formation was observed in cells transduced with the three miRNAs (Supplementary Figure S2C). To further confirm the specificity of the three miRNAs, we transfected the coding sequence of GRP78 lacking its 3′UTR into C42B cells stably expressing miR-30d, miR-181a and miR-199a-5p. Our data showed that, after transfection, GRP78 expression was restored at both mRNA and protein levels, and that the inhibitory effects of the three miRNAs on cell survival and colony formation were abrogated, suggesting that miR-30d, miR-181a and miR-199a-5p require the 3′UTR region of GRP78 to exert their actions (Supplementary Figures S3A–D). Their binding is lost upon overexpressing GRP78 lacking the 3′UTR sequence, which is consistent with mutation of their binding sites in the luciferase assay (Figure 2). Taken together, these results indicate that the cooperative effect of multiple miRNAs that target GRP78 is specific and maintained in a stable expression system. The results also suggest that the magnitude of the overall effect of decreasing GRP78 levels is cell-type specific.
Lentiviral delivery of multiple co-transcribed miRNAs down-regulates GRP78 protein levels, reduces cell viability and induces apoptosis in different cancer cell lines. (a) Expression vectors were generated by cloning miR-30d, miR-181a and miR-199a-5p into a lentiviral vector. (b) GRP78 protein levels were determined by western blot in C42B cells infected with lentivirus vectors only (LV) or containing multiple miRNAs (LV miR-30+181+199). Protein levels were normalized to Actin. (c–e) C42B, HCT116 and HL-60 cells infected with LV or LV miR-30+181+199 were treated with Tg, or the histone deacetylase inhibitor (TSA). GRP78 and PARP-1 protein levels were determined by western blot. Total cell numbers were counted 4 days after the treatment. Data represent mean±s.d. (n=3). *P<0.05.
MiR-30d, miR-181a and miR-199a-5p inhibit tumor growth in vivo
To investigate the potential anti-tumor activity of these three miRNAs in vivo, cells stably expressing miR-30d, miR-181a and miR-199a-5p were cultured in a three-dimensional matrix (transglutaminase-gelatin gel) to mimic the tumor environment and then injected subcutaneously into athymic nude male mice (n=6 in each control and experimental group). Tumors were allowed to grow for 45 days. HCT116 successfully formed tumors, whereas HCT116 expressing miR-30d, miR-181a and miR-199a-5p showed tumors that were two- to threefold smaller in size than those of control cells, suggesting that the three miRNAs inhibit tumorigenesis in vivo (Supplementary Figures S4A and B). In mice injected with C42B cells, angiogenesis was observed but no tumor formation was detected. After necropsy, we found that some cells were still trapped in the gel matrix (data not shown). A possible explanation for these findings is that C42B cells may require longer time for tumor formation when injected subcutaneously.38 C42B cells stably expressing the three miRNAs did not form tumors. Small nodules, and cells trapped in the gel matrix were observed in mice injected with HL-60 cells, whereas no nodules were observed in HL-60 cells stably expressing miR-30d, miR-181a and miR-199a-5p (data not shown). Since HL-60 was derived from a liquid tumor, we cannot rule out the possibility that cell may have migrated to the lymph nodes. Further studies will help clarify this point.
Discussion
GRP78 up-regulation in various tumor types and its induction after drug treatment has been shown to be a major contributor to tumorigenesis and therapeutic resistance.1, 39 Despite advances in our understanding of GRP78 actions and its induction by ER stress, little is known about endogenous inhibitors controlling its expression other than repression of its basal expression by histone deacetylases.16 Thus, discovery of such regulatory moieties will be important for designing more efficacious therapeutic approaches. In our study, we identify miR-30d, miR-181a and miR-199a-5p as regulatory small RNAs that act cooperatively to control GRP78 levels. This effect cannot be attributed to the miRNA dose, since the same amount of total miRNA was used irrespective of whether they were transfected alone or in combination. Because the reduction in GRP78 levels occurs at both mRNA and protein levels, it is likely that the three miRNAs used in our study act through GRP78 mRNA destabilization and not translational repression.40 The fact that miR-30d, miR-181a and miR-199a-5p are down-regulated and GRP78 is up-regulated in samples of prostate, colon and bladder cancer patients, suggests that these miRNAs are clinically relevant and that all three miRNAs are required to suppress GRP78.41 Roue et al.15 demonstrated that inhibition of GRP78 using siRNA can overcome drug resistance in mantle cell lymphoma. In our study, we show that decreased levels of GRP78 achieved by either transient transfection or transduction of multiple miRNAs can also increase the sensitivity of cancer cells to TSA treatment, resulting in the induction of apoptosis, and inhibition of cell growth and colony formation. Therefore, the use of multiple specific miRNAs to target key genes involved in tumorigenesis could provide an exciting avenue for the development of new cancer therapies. Our results also suggest that co-expression of multiple miRNAs from a single lentiviral vector platform42 is an efficient method to deliver and test the putative combinatorial actions of miRNAs on a single target gene. Moreover, in vivo data show that the tumor size of the HCT116 stably expressing miR-30d, miR-181a and miR-199a-5p is significantly smaller than that of control cells, consistent with the notion that these miRNAs suppress GRP78 expression, leading to inhibition of tumor growth. However, the effect of the miRNAs on their targets within the tumor microenvironment requires further investigation.
The critical role of miRNAs in cancer and their involvement in common cellular pathways make them valuable and comprehensive targets.27 In addition to targeting GRP78, miR-30d can act as a negative regulator for p53 and regulate cell-cycle arrest and apoptosis,43 while down-regulation of miR-199a-5p in human hepatocellular carcinoma is highly associated with cell invasion.44 On the other hand, it is well established that a single mRNA molecule can be targeted by multiple miRNAs, and studies focusing on the combinatorial actions of miRNAs have begun to emerge.45, 46, 47, 48 For instance, multiple miRNAs have been recently shown to regulate PTEN expression in T-cell lymphoblastic leukemia cells.47 Such studies indicate that gene expression is tightly controlled by miRNA networks.49 The results presented here suggest that the combined action of multiple miRNAs might be essential to achieve efficient down-regulation of GRP78. If this holds true for other genes, it may help explain why the validation of the majority of miRNA targets found using prediction algorithms, which is based on single miRNA-based experiments, has been unsuccessful.50, 51
In conclusion, we report that miR-30d, miR-181a and miR-199a-5p regulate GRP78 and that their decreased expression in tumor cells results in increased GRP78 levels, which in turn promotes tumorigenesis and therapeutic resistance. To the best of our knowledge, this is the first report identifying the specific miRNAs that repress GRP78 and their combinatorial regulatory action. Notably, our results suggest that the use of miRNAs and TSA or other therapeutic agents in combination therapy may provide a powerful approach in the treatment of GRP78-overexpressing and drug-resistant tumors.
Materials and methods
Cell lines, primary tumors and drugs
Normal fibroblast LD419, non-tumorigenic human urothelial UROtsa and NK2464 cells were obtained and cultured as described previously.52 C42B cells were a gift from Dr M Stallcup (USC) and were maintained in RPMI supplemented with 10% fetal bovine serum. UM-UC-3, J82, HCT116, HL-60 cells were obtained from ATCC (Manassas, VA, USA) and cultured according to the recommended protocols. Patient samples were obtained through University of Southern California/Norris Tissue Procurement Core Resource after informed consent and Institutional Review Board approval at the University of Southern California/Norris Comprehensive Cancer Center. Tg and TSA (Sigma-Aldrich, St Louis, MO, USA) were prepared as previously described.16
Reverse transcription and quantitative real-time PCR analysis
MiRNA Taqman assays (Applied Biosystems, Foster City, CA, USA) were performed following the manufacturer’s instructions. Total RNA (15 ng) was reverse transcribed into miR- and U6-specific cDNA and miRNA expression was normalized to U6 snRNA. The mRNA levels were measured by real-time PCR as described.29 Total RNA was extracted by Trizol (Invitrogen, Carlsbad, CA, USA) and cDNA was prepared by M-MLV reversed transcriptase and random hexamers (Promega, Madison, WI, USA). The miRNA expression was normalized to human GAPDH (glyceraldehyde-3-phosphate dehydrogenase, one of the most commonly used housekeeping genes).
Western blot assay
The same amount of total cell lysates were prepared for western blot analysis as described.29 Antibodies against GRP78 (BD Pharmingen, San Jose, CA, USA); CHOP protein, PARP-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA); β-actin (Sigma) were used.
Transfection
MiRNA precursors (Ambion, Foster City, CA, USA) were transfected into cells at the final concentration of 50 nM using Oligofectamine (Invitrogen) following the manufacturer’s protocol.
Generation of GRP78 3′UTR luciferase constructs
The human GRP78 3′UTR (387 bp) was cloned into the XbaI site of pGL3-control vector (Promega). Mutant vectors were generated using designed mutagenic oligonucleotide primers by the QuikChange II XL site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). Each miRNA-binding site mutation was carried out by one complete procedure of mutant synthesis.
Luciferase reporter assay
Luciferase reporter vectors, either WT or mutant (500 ng), together with pRL-SV40 (5 ng) (Promega) were co-transfected with miRNA precursor at the final concentration of 150 nM using Lipofectamine 2000 (Invitrogen). Luciferase activity was measured using the Dual Luciferase assay kit (Promega) according to the manufacturer’s instructions. Firefly luciferase activity was normalized to the internal control Renilla.
Apoptosis assay
Annexin V-FITC apoptosis detection kit (Medical & Biological Laboratories, Woburn, MA, USA) was used according to the manufacturer’s instructions. Briefly, both attached and floating cells were collected and resuspended in binding buffer before adding the Annexin V-FITC antibody and propidium iodide. Stained cells were analyzed by flow cytometry (Beckman Coulter, Brea, CA, USA).
Cell viability assay and colony formation assay
Cell viability assay and colony formation were described previously.29 Total cell numbers were counted at the indicated time points. In all, 1000 of cells were seeded and incubated at 37 °C, 5% CO2 for 14 days to allow colonies to form. Colonies were fixed in methanol, stained with 10% of Giemsa solution (Sigma) and counted.
Expression vectors and virus transduction
Expression vectors were made by sequentially cloning each of the precursor miRNA into pcDNA3.1(+) (Invitrogen). MiRNAs were then cloned into the lentivirus vector using In-Fusion Advantage PCR cloning kit (Clontech, Mountain View, CA, USA). Cells were infected with RNA virus stock in the presence of polybrene (70 μg/ml). After 24 h, the medium was substituted with fresh medium containing puromycin (1.25 μg/ml). After 7 days of selection, the puromycin was removed from the medium.
In vivo cell injection with 3D gelatin-TGase
Athymic nude male mice (25–35 g) were used in the study according to an approved protocol by the Institutional Animal Care and Committee, University of Southern California. Animals were anesthetized with ketamine/xylazine (10:1,w/w) before the injection. Gelatin-TGase was prepared as previously described.53 About 200 μl of gelatin-TGase cocktail mixed with 106 cells was injected through a 27-gauge needle on the shank of the mouse. Tumors were allowed to grow for 45 days, after which time, mice were necropsied and tumor size was measured.
References
Lee AS . GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res 2007; 67: 3496–3499.
Pfaffenbach KT, Lee AS . The critical role of GRP78 in physiologic and pathologic stress. Curr Opin Cell Biol 2011; 23: 150–156.
Ron D, Walter P . Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007; 8: 519–529.
Kahali S, Sarcar B, Prabhu A, Seto E, Chinnaiyan P . Class I histone deacetylases localize to the endoplasmic reticulum and modulate the unfolded protein response. FASEB J 2012; 26: 2437–2445.
Ni M, Zhang Y, Lee AS . Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem J 2011; 434: 181–188.
Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science 2006; 312: 572–576.
Dong D, Stapleton C, Luo B, Xiong S, Ye W, Zhang Y et al. A critical role for GRP78/BiP in the tumor microenvironment for neovascularization during tumor growth and metastasis. Cancer Res 2011; 71: 2848–2857.
Luo B, Lee AS . The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene 2013; 32: 805–818.
Yeung BH, Kwan BW, He QY, Lee AS, Liu J, Wong AS . Glucose-regulated protein 78 as a novel effector of BRCA1 for inhibiting stress-induced apoptosis. Oncogene 2008; 27: 6782–6789.
Grkovic S, O'Reilly VC, Han S, Hong M, Baxter RC, Firth SM . IGFBP-3 binds GRP78, stimulates autophagy and promotes the survival of breast cancer cells exposed to adverse microenvironments. Oncogene 2013; 32: 2412–2420.
Li N, Zoubeidi A, Beraldi E, Gleave ME . GRP78 regulates clusterin stability, retrotranslocation and mitochondrial localization under ER stress in prostate cancer. Oncogene 2013; 32: 1933–1942.
Pootrakul L, Datar RH, Shi SR, Cai J, Hawes D, Groshen SG et al. Expression of stress response protein Grp78 is associated with the development of castration-resistant prostate cancer. Clin Cancer Res 2006; 12 (20 Pt 1): 5987–5993.
Wey S, Luo B, Tseng CC, Ni M, Zhou H, Fu Y et al. Inducible knockout of GRP78/BiP in the hematopoietic system suppresses Pten-null leukemogenesis and AKT oncogenic signaling. Blood 2011; 119: 817–825.
Fu Y, Wey S, Wang M, Ye R, Liao CP, Roy-Burman P et al. Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of ER chaperone GRP78/BiP in prostate epithelium. Proc Natl Acad Sci USA 2008; 105: 19444–19449.
Roue G, Perez-Galan P, Mozos A, Lopez-Guerra M, Xargay-Torrent S, Rosich L et al. The Hsp90 inhibitor IPI-504 overcomes bortezomib resistance in mantle cell lymphoma in vitro and in vivo by down-regulation of the prosurvival ER chaperone BiP/Grp78. Blood 2011; 117: 1270–1279.
Baumeister P, Dong D, Fu Y, Lee AS . Transcriptional induction of GRP78/BiP by histone deacetylase inhibitors and resistance to histone deacetylase inhibitor-induced apoptosis. Mol Cancer Ther 2009; 8: 1086–1094.
Pyrko P, Schönthal AH, Hofman FM, Chen TC, Lee AS . The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res 2007; 67: 9809–9816.
Virrey JJ, Dong D, Stiles C, Patterson JB, Pen L, Ni M et al. Stress chaperone GRP78/BiP confers chemoresistance to tumor-associated endothelial cells. Mol Cancer Res 2008; 6: 1268–1275.
Backer MV, Backer JM, Chinnaiyan P . Targeting the unfolded protein response in cancer therapy. Methods Enzymol 2011; 491: 37–56.
Lee RC, Feinbaum RL, Ambros V . The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993; 75: 843–854.
Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ . miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res 2006; 34 (Database issue): D140–D144.
Ambros V . The functions of animal microRNAs. Nature 2004; 431: 350–355.
Krol J, Loedige I, Filipowicz W . The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 2010; 11: 597–610.
Croce CM . Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet 2009; 10: 704–714.
Calin GA, Croce CM . MicroRNA signatures in human cancers. Nat Rev Cancer 2006; 6: 857–866.
Kappelmann M, Kuphal S, Meister G, Vardimon L, Bosserhoff A-K . MicroRNA miR-125b controls melanoma progression by direct regulation of c-Jun protein expression. Oncogene 2013; 32: 2984–2991.
Garzon R, Marcucci G, Croce CM . Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov 2010; 9: 775–789.
Saito Y, Liang G, Egger G, Friedman JM, Chuang JC, Coetzee GA et al. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell 2006; 9: 435–443.
Friedman JM, Liang G, Liu CC, Wolff EM, Tsai YC, Ye W et al. The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res 2009; 69: 2623–2629.
Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA 2004; 101: 2999–3004.
Lopez-Serra P, Esteller M . DNA methylation-associated silencing of tumor-suppressor microRNAs in cancer. Oncogene 2012; 31: 1609–1622.
Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA 2002; 99: 15524–15529.
Hu W, Chan CS, Wu R, Zhang C, Sun Y, Song JS et al. Negative regulation of tumor suppressor p53 by microRNA miR-504. Mol Cell 2010; 38: 689–699.
Zhang Y, Roccaro AM, Rombaoa C, Flores L, Obad S, Fernandes SM et al. LNA-mediated anti-microRNA-155 silencing in low-grade B cell lymphomas. Blood 2012; 120: 1678–1686.
Leung AK, Sharp PA . MicroRNA functions in stress responses. Mol Cell 2010; 40: 205–215.
Duan Q, Wang X, Gong W, Ni L, Chen C, He X et al. ER stress negatively modulates the expression of the miR-199a/214 cluster to regulates tumor survival and progression in human hepatocellular cancer. PLoS One 2012; 7: e31518.
Cook KL, Shajahan AN, Wärri A, Jin L, Hilakivi-Clarke LA, Clarke R . Glucose-regulated protein 78 controls cross-talk between apoptosis and autophagy to determine antiestrogen responsiveness. Cancer Res 2012; 72: 3337–3349.
Zhang J, Dai J, Qi Y, Lin DL, Smith P, Strayhorn C et al. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Invest 2001; 107: 1235–1244.
Booth L, Cazanave SC, Hamed HA, Yacoub A, Ogretmen B, Chen CS et al. OSU-03012 suppresses GRP78/BiP expression that causes PERK-dependent increases in tumor cell killing. Cancer Biol Ther 2012; 13: 224–236.
Guo H, Ingolia NT, Weissman JS, Bartel DP . Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010; 466: 835–840.
Daneshmand S, Quek ML, Lin E, Lee C, Cote RJ, Hawes D et al. Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum Pathol 2007; 38: 1547–1552.
Qiu X, Friedman JM, Liang G . Creating a flexible multiple microRNA expression vector by linking precursor microRNAs. Biochem Biophys Res Commun 2011; 411: 276–280.
Kumar M, Lu Z, Takwi AA, Chen W, Callander NS, Ramos KS et al. Negative regulation of the tumor suppressor p53 gene by microRNAs. Oncogene 2011; 30: 843–853.
Shen Q, Cicinnati VR, Zhang X, Iacob S, Weber F, Sotiropoulos GC et al. Role of microRNA-199a-5p and discoidin domain receptor 1 in human hepatocellular carcinoma invasion. Mol Cancer 2010; 9: 227.
Lewis BP, Burge CB, Bartel DP . Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005; 120: 15–20.
Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005; 433: 769–773.
Mavrakis KJ, Van Der Meulen J, Wolfe AL, Liu X, Mets E, Taghon T et al. A cooperative microRNA-tumor suppressor gene network in acute T-cell lymphoblastic leukemia (T-ALL). Nat Genet 2011; 43: 673–678.
Marasa BS, Srikantan S, Masuda K, Abdelmohsen K, Kuwano Y, Yang X et al. Increased MKK4 abundance with replicative senescence is linked to the joint reduction of multiple microRNAs. Sci Signal 2009; 2: ra69.
Mavrakis KJ, Leslie CS, Wendel HG . Cooperative control of tumor suppressor genes by a network of oncogenic microRNAs. Cell Cycle 2011; 10: 2845–2849.
Bartel DP . MicroRNAs: target recognition and regulatory functions. Cell 2009; 136: 215–233.
Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N . Widespread changes in protein synthesis induced by microRNAs. Nature 2008; 455: 58–63.
Wolff EM, Byun HM, Han HF, Sharma S, Nichols PW, Siegmund KD et al. Hypomethylation of a LINE-1 promoter activates an alternate transcript of the MET oncogene in bladders with cancer. PLoS Genet 2010; 6: e1000917.
Kuwahara K, Yang Z, Slack GC, Nimni ME, Han B . Cell delivery using an injectable and adhesive transglutaminase-gelatin gel. Tissue Eng Part C Methods 2010; 16: 609–618.
Acknowledgements
We thank Dr PA Jones for supporting this work; Dr KT Pfaffenbach for the experimental suggestions; Drs J Zhou and Q Li for data analysis; Drs TK Kelly for careful reading of the manuscript; Ms K Pandiyan and Mr C Duymich for the overview. This work was supported by NCI Grants (RO1 CA138794, GL, RO1 CA027607, ASL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Su, SF., Chang, YW., Andreu-Vieyra, C. et al. miR-30d, miR-181a and miR-199a-5p cooperatively suppress the endoplasmic reticulum chaperone and signaling regulator GRP78 in cancer. Oncogene 32, 4694–4701 (2013). https://doi.org/10.1038/onc.2012.483
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.483
Keywords
This article is cited by
-
Abiraterone induces SLCO1B3 expression in prostate cancer via microRNA-579-3p
Scientific Reports (2021)
-
Interplay between endoplasmic reticulum stress and non-coding RNAs in cancer
Journal of Hematology & Oncology (2020)
-
MicroRNAs and obesity-induced endothelial dysfunction: key paradigms in molecular therapy
Cardiovascular Diabetology (2020)
-
Therapeutic potential of KLF2-induced exosomal microRNAs in pulmonary hypertension
Nature Communications (2020)
-
Angiotensin II Increases Endoplasmic Reticulum Stress in Adipose Tissue and Adipocytes
Scientific Reports (2019)