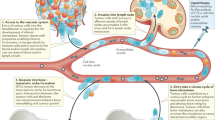
Abstract
Although the survival rate for early detected cancers is high, once a cancer metastasizes to bone, it is incurable. Interestingly, patients without visible metastases display abnormal bone formation and resorption, suggesting a link between primary cancers and the bone microenvironment prior to metastasis, and this link likely facilitates preparation of the pre-metastatic niche. We hypothesized that communication with the primary tumor would result in bone remodeling alterations, and that platelets could facilitate this communication. By using three tumor models, we demonstrate that primary tumor growth stimulates bone formation measured by microcomputed tomography. Further, platelet depletion prevented tumor-induced bone formation, highlighting the importance of platelets in the communication between tumors and the bone microenvironment. Finally, we determine that platelets sequester a variety of tumor-derived proteins, TGF-β1 and MMP-1 in particular, which regulate bone formation. Thus, our data reveal that platelets function as mediators of tumor–bone communication prior to metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Abbreviations
- BV/TV:
-
ratio of bone volume to total volume
- microCT:
-
microcomputed tomography
- Tb.N:
-
trabecular number
- TIMP:
-
tissue inhibitor of metalloproteinase
References
American Cancer Society Prostate Cancer. American Cancer Society, Atlanta, GA, 2008.
American Cancer Society Melanoma Skin Cancer. American Cancer Society, Atlanta, GA, 2008.
Coleman RE, Guise TA, Lipton A, Roodman GD, Berenson JR, Body J-J et al. Advancing Treatment for Metastatic Bone Cancer. Clin Cancer Res 2008; 14: 6387–6395.
Siegel R, Ward E, Brawley O, Jemal A . Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011; 61: 212–236.
Kingsley LA, Fournier PGJ, Chirgwin JM, Guise TA . Molecular Biology of Bone Metastasis. Mol Cancer Ther 2007; 6: 2609–2617.
Mundy GR . Metastasis to Bone: Causes Consequences and Therapeutic Opportunities. Nat Rev Cancer 2002; 2: 584–593.
Karpatkin S, Pearlstein E, Ambrogio C, Coller BS . Role of Adhesive Proteins in Platelet Tumor Interaction In Vitro and Metastasis Formation In Vivo. J Clin Invest 1988; 81: 1012–1019.
Borsig L . The Role of Platelet Activation in Tumor Metastasis. Expert Rev Anticancer Ther 2008; 8: 1247–1255.
Feng W, Madajka M, Kerr BA, Mahabeleshwar GH, Whiteheart SW, Byzova TV . A novel role for platelet secretion in angiogenesis: mediating bone marrow-derived cell mobilization and homing. Blood 2011; 117: 3893–3902.
Kawasumi M, Kitoh H, Siwicka KA, Ishiguro N . The Effect of the Platelet Concentration in Platelet-Rich Plasma Gel on the Regeneration of Bone. J Bone Joint Surg Br 2007; 90-B: 966–972.
Uggeri J, Belletti S, Guizzardi S, Poli T, Cantarelli S, Scandroglio R et al. Dose-Dependent Effects of Platelet Gel Releasate on Activites of Human Osteoblasts. J Periodontol 2007; 78: 1985–1991.
Gruber R, Karreth F, Fischer MB, Watzek G . Platelet-Released Supernatants Stimulate Formation of Osteoclast-like Cells through a Prostaglandin/RANKL-Dependent Mechanism. Bone 2002; 30: 726–732.
Nair MB, Varma HK, John A . Platelet-Rich Plasma and Fibrin Glue-Coated Bioactive Ceramics Enhance Growth and Differentiation of Goat Bone Marrow-Derived Stem Cells. Tissue Eng Part A 2009; 15: 1619–1631.
McCabe NP, Madajka M, Vasanji A, Byzova TV . Intraosseous Injection of RM1 Murine Prostate Cancer Cells Promotes Rapid Osteolysis and Periosteal Bone Deposition. Clin Exp Metastasis 2008; 25: 581–590.
McCabe NP, De S, Vasanji A, Brainard J, Byzova TV . Prostate Cancer Specific Integrin αvβ3 Modulates Bone Metastatic Growth and Tissue Remodeling. Oncogene 2007; 26: 6238–6243.
McCabe NP, Kerr BA, Madajka M, Vasanji A, Byzova TV . Augmented Osteolysis in SPARC-Deficient Mice with Bone-Residing Prostate Cancer. Neoplasia 2011; 13: 31–39.
Gomes RR Jr, Buttke P, Paul EM, Sikes RA . Osteosclerotic prostate cancer metastasis to murine bone are enhanced with increased bone formation. Clin Exp Metastasis 2009; 26: 641–651.
Edlund M, Sung S-Y, Chung LW . Modulation of Prostate Cancer Growth in Bone Microenvironments. J Cell Biochem 2004; 91: 686–705.
Roudier MP, Morrissey C, True LD, Higano CS, Vessella RL, Ott SM . Histopathological Assessment of Prostate Cancer Bone Osteoblastic Metastases. J Urol 2008; 180: 1154–1160.
Feng W, McCabe NP, Mahabeleshwar GH, Somanath PR, Phillips DR, Byzova TV . The Angiogenic Response Is Dictated by β3 Integrin on Bone Marrow-Derived Cells. J Cell Biol 2008; 183: 1145–1157.
Rhee JS, Black M, Schubert U, Fischer S, Morgenstern E, Hammes HP et al. The functional role of blood platelet components in angiogenesis. Thromb Haemost 2004; 92: 394–402.
Gasic GJ, Gasic TB, Stewart CC . Antimetastatic Effects Associated with Platelet Reduction. Proc Natl Acad Sci USA 1968; 61: 46–52.
Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A . Heparin and Cancer Revisited: Mechanistic Connections Involving Platelets, P-selectin, Carcinoma Mucins, and Tumor Metastasis. Proc Natl Acad Sci USA 2001; 98: 3352–3357.
Oprea WE, Karp JM, Hosseini MM, Davies JE . Effect of platelet releasate on bone cell migration and recruitment in vitro. J Craniofac Surg 2003; 14: 292–300.
Mishra A, Tummala P, King A, Lee B, Kraus M, Tse V et al. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng Part C Methods 2009; 15: 431–435.
Italiano JE Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S et al. Angiogenesis Is Regulated by a Novel Mechanism: Pro- and Antiangiogenic Proteins Are Organized into Separate Platelet α Granules and Differentially Released. Blood 2008; 111: 1227–1233.
Klement GL, Yip T-T, Cassiola F, Kikuchi L, Cervi D, Podust VN et al. Platelets Actively Sequester Angiogenesis Regulators. Blood 2009; 113: 2835–2842.
Baselga J, Rothenberg ML, Tabernero J, Seoane J, Daly T, Cleverly A et al. TGF-β Signalling-Related Markers in Cancer Patients with Bone Metastasis. Biomarkers 2008; 13: 217–236.
Shariat SF, Shalev M, Menesses-Diaz A, Kim IY, Kattan MW, Wheeler TM et al. Preoperative Plasma Levels of Transforming Growth Factor Beta1 (TGF-β1) Strongly Predict Progression in Patients Undergoing Radical Prostatectomy. J Clin Oncol 2001; 19: 2856–2864.
Stellos K, Gawaz M . Platelet Interaction with Progenitor Cells: Potential Implications for Regenerative Medicine. Thromb Haemost 2007; 98: 922–929.
Schultz-Cherry S, Murphy-Ullrich JE . Thrombospondin Causes Activation of Latent Transforming Growth Factor-β Secreted by Endothelial Cells by a Novel Mechanism. J Cell Biol 1993; 122: 923–932.
Street J, Bao M, deGuzman L, Bunting S, Peale FV Jr, Ferrara N et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA 2002; 99: 9656–9661.
Joyce ME, Roberts AB, Sporn MB, Bolander ME . Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol 1990; 110: 2195–2207.
Saadeh PB, Mehrara BJ, Steinbrech DS, Dudziak ME, Greenwald JA, Luchs JS et al. Transforming growth factor-beta1 modulates the expression of vascular endothelial growth factor by osteoblasts. Am J Physiol 1999; 277 (4 Pt 1): C628–C637.
Zelzer E, McLean W, Ng YS, Fukai N, Reginato AM, Lovejoy S et al. Skeletal defects in VEGF(120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development 2002; 129: 1893–1904.
Sims NA, Gooi JH . Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol 2008; 19: 444–451.
Haeusler G, Walter I, Helmreich M, Egerbacher M . Localization of matrix metalloproteinases, (MMPs) their tissue inhibitors, and vascular endothelial growth factor (VEGF) in growth plates of children and adolescents indicates a role for MMPs in human postnatal growth and skeletal maturation. Calcif Tissue Int 2005; 76: 326–335.
Blouin S, Baslé MF, Chappard D . Interactions Between Microenvironment and Cancer Cells in Two Animal Models of Bone Metastasis. Br J Cancer 2008; 98: 809–815.
Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I et al. Regulation of Cancer Cell Migration and Bone Metastasis by RANKL. Nature 2006; 440: 692–696.
Kerr BA, Miocinovic R, Smith AK, Klein EA, Byzova TV . Comparison of Tumor and Microenvironment Secretomes in Plasma and in Platelets during Prostate Cancer Growth in a Xenograft Model. Neoplasia 2010; 12: 388–396.
Kerr BA, Byzova TV . MicroCT: An Essential Tool in Bone Metastasis Research. In: Saba L, (ed). Computed Tomography - Clinical Applications. InTech, New York, USA, 2012, pp 211–230.
Reinholz GG, Getz B, Pederson L, Sanders ES, Subramaniam M, Ingle JN et al. Bisphosphonates Directly Regulate Cell Proliferation, Differentiation, and Gene Expression in Human Osteoblasts. Cancer Res 2000; 60: 6001–6007.
Acknowledgements
We thank Miroslava Tischenko, Steven Maximuk and Richard Rozic for their technical assistance. Dr Amit Vasanji developed the software and techniques for the microCT analysis. Cleveland Clinic Biomedical Imaging and Analysis Core Center was funded, in part by NIAMS Core Center, grant no. 1P30 AR050953. BAK was supported by a Ruth L Kirschstein NRSA award (F32 CA142133) from the NIH/NCI. NPM was supported by a Ruth L Kirstein NRSA award (F32 CA117262). This study was supported by research funding from the NIH/NCI (grant no. CA126847) to TVB.
Author contributions: BAK designed and performed the research, analyzed the data and wrote the paper; NPM and WF performed the research and analyzed the data; TVB designed the research, analyzed the data and wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Byzova’s work has been funded by the NIH. Dr Kerr and Dr McCabe have been awarded fellowships from the NIH. Dr W Feng declares no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Kerr, B., McCabe, N., Feng, W. et al. Platelets govern pre-metastatic tumor communication to bone. Oncogene 32, 4319–4324 (2013). https://doi.org/10.1038/onc.2012.447
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.447
Keywords
This article is cited by
-
The dynamic role of platelets in cancer progression and their therapeutic implications
Nature Reviews Cancer (2024)
-
Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments
Molecular Cancer (2022)
-
Platelets, immune cells and the coagulation cascade; friend or foe of the circulating tumour cell?
Molecular Cancer (2021)
-
Diagnosis of thyroid neoplasm using support vector machine algorithms based on platelet RNA-seq
Endocrine (2021)
-
Ticagrelor inhibits platelet–tumor cell interactions and metastasis in human and murine breast cancer
Clinical & Experimental Metastasis (2018)